Page 179 - Chiral Separation Techniques
P. 179
6.3 Structure–Binding Relationships 157
6.3 Structure-Binding Relationships
A large number of racemates have been successfully resolved on tailor-made MIC-
SPs (Table 6-1). Using MAA as functional monomer, good recognition is obtained
for templates containing Brönsted-basic or hydrogen bonding functional groups
close to the stereogenic center. On the other hand, templates containing acidic func-
tional groups are better imprinted using a basic functional monomer such as
vinylpyridine. This emphasizes the importance of functional group complementarity
when designing the MICSPs. Furthermore, the separation factors are high and higher
than those observed for many of the widely used commercial CSPs [20]. However,
the columns are tailor-made and the number of racemates resolved equals nearly the
number of stationary phases, i.e. each column can resolve only a limited number of
racemates. Although the separation factors are high, the resolution factors are low,
but the performance can often be enhanced by running the separations at higher tem-
peratures [15] and by switching to an aqueous mobile phase (Fig. 6-3) [21], or by
performing the imprinting in situ in fused silica capillaries for use in capillary elec-
trochromatography [22, 23]. At low sample loads, the retention on the MICSPs is
extremely sensitive to the amount of sample injected, indicating overloading of a
small amount of high energy binding sites. [24] Moreover the peaks corresponding
to the template are usually broad and asymmetric. This is ascribed to the mentioned
site heterogeneity together with a slow mass transfer (see Section 6.3.1).
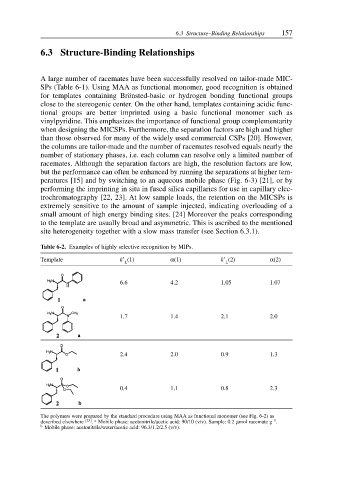
Table 6-2. Examples of highly selective recognition by MIPs.
Template k’ (1) α(1) k’ (2) α(2)
L L
6.6 4.2 1.05 1.07
1.7 1.4 2.1 2.0
2.4 2.0 0.9 1.3
0.4 1.1 0.8 2.3
The polymers were prepared by the standard procedure using MAA as functional monomer (see Fig. 6-2) as
described elsewhere [25] a –1
. Mobile phase: acetonitrile/acetic acid: 90/10 (v/v). Sample: 0.2 µmol racemate g .
b Mobile phase: acetonitrile/water/acetic acid: 96.3/1.2/2.5 (v/v).