Page 180 - Chiral Separation Techniques
P. 180
158 6 Enantiomer Separations using Designed Imprinted Chiral Phases
6.3.1 High Selectivity
MICSPs are often highly selective for their respective template molecule. This was
the case for polymer imprinted with L-phenylalanine anilide (L-PA) and L-pheny-
lalanine-N-methylanilide respectively (comparing a secondary and tertiary amide as
template) (Table 6-2) [25]. The racemate corresponding to the template was well
resolved on the corresponding MICSP, whereas the analogue racemate was less
retained and only poorly resolved. Similar results were obtained when comparing a
polymer imprinted with L-phenylalanine ethyl ester and one with its phosphonate
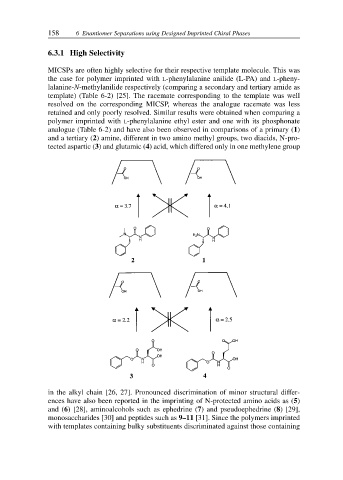
analogue (Table 6-2) and have also been observed in comparisons of a primary (1)
and a tertiary (2) amine, different in two amino methyl groups, two diacids, N-pro-
tected aspartic (3) and glutamic (4) acid, which differed only in one methylene group
in the alkyl chain [26, 27]. Pronounced discrimination of minor structural differ-
ences have also been reported in the imprinting of N-protected amino acids as (5)
and (6) [28], aminoalcohols such as ephedrine (7) and pseudoephedrine (8) [29],
monosaccharides [30] and peptides such as 9–11 [31]. Since the polymers imprinted
with templates containing bulky substituents discriminated against those containing