Page 177 - Chiral Separation Techniques
P. 177
6.2 Molecular Imprinting Approaches 155
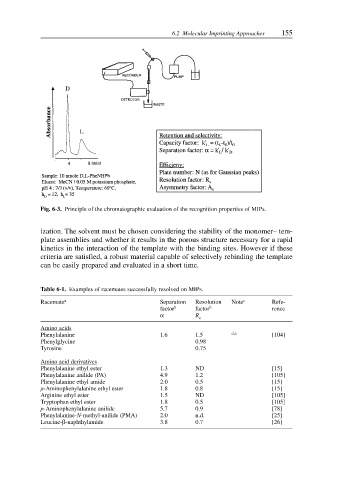
Fig. 6-3. Principle of the chromatographic evaluation of the recognition properties of MIPs.
ization. The solvent must be chosen considering the stability of the monomer– tem-
plate assemblies and whether it results in the porous structure necessary for a rapid
kinetics in the interaction of the template with the binding sites. However if these
criteria are satisfied, a robust material capable of selectively rebinding the template
can be easily prepared and evaluated in a short time.
Table 6-1. Examples of racemates successfully resolved on MIPs.
Racemate a Separation Resolution Note c Refe-
factor b factor b rence
α R
s
Amino acids
Phenylalanine 1.6 1.5 d,e [104]
Phenylglycine 0.98
Tyrosine 0.75
Amino acid derivatives
Phenylalanine ethyl ester 1.3 ND [15]
Phenylalanine anilide (PA) 4.9 1.2 [105]
Phenylalanine ethyl amide 2.0 0.5 [15]
p-Aminophenylalanine ethyl ester 1.8 0.8 [15]
Arginine ethyl ester 1.5 ND [105]
Tryptophan ethyl ester 1.8 0.5 [105]
p-Aminophenylalanine anilide 5.7 0.9 [78]
Phenylalanine-N-methyl-anilide (PMA) 2.0 n.d. [25]
Leucine-β-naphthylamide 3.8 0.7 [26]