Page 176 - Chiral Separation Techniques
P. 176
154 6 Enantiomer Separations using Designed Imprinted Chiral Phases
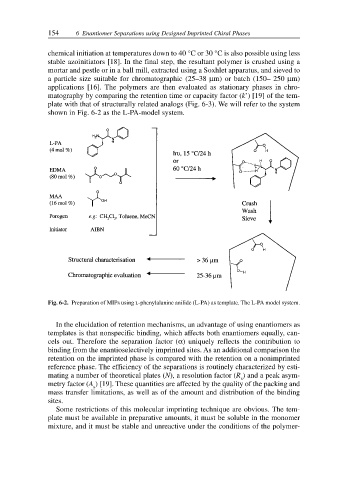
chemical initiation at temperatures down to 40 °C or 30 °C is also possible using less
stable azoinitiators [18]. In the final step, the resultant polymer is crushed using a
mortar and pestle or in a ball mill, extracted using a Soxhlet apparatus, and sieved to
a particle size suitable for chromatographic (25–38 µm) or batch (150– 250 µm)
applications [16]. The polymers are then evaluated as stationary phases in chro-
matography by comparing the retention time or capacity factor (k’) [19] of the tem-
plate with that of structurally related analogs (Fig. 6-3). We will refer to the system
shown in Fig. 6-2 as the L-PA-model system.
Fig. 6-2. Preparation of MIPs using L-phenylalanine anilide (L-PA) as template. The L-PA model system.
In the elucidation of retention mechanisms, an advantage of using enantiomers as
templates is that nonspecific binding, which affects both enantiomers equally, can-
cels out. Therefore the separation factor (α) uniquely reflects the contribution to
binding from the enantioselectively imprinted sites. As an additional comparison the
retention on the imprinted phase is compared with the retention on a nonimprinted
reference phase. The efficiency of the separations is routinely characterized by esti-
mating a number of theoretical plates (N), a resolution factor (R ) and a peak asym-
s
metry factor (A ) [19]. These quantities are affected by the quality of the packing and
s
mass transfer limitations, as well as of the amount and distribution of the binding
sites.
Some restrictions of this molecular imprinting technique are obvious. The tem-
plate must be available in preparative amounts, it must be soluble in the monomer
mixture, and it must be stable and unreactive under the conditions of the polymer-