Page 237 - Chiral Separation Techniques
P. 237
8.5 Experimental Examples of Separations 215
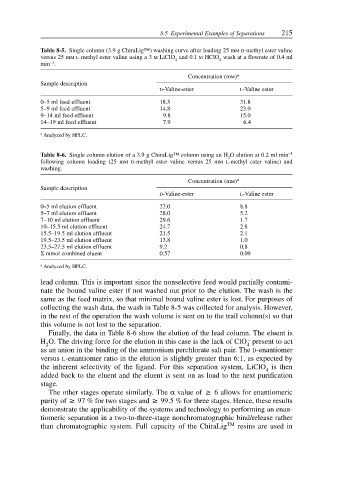
Table 8-5. Single column (3.9 g ChiraLig™) washing curve after loading 25 mMD-methyl ester valine
versus 25 mML-methyl ester valine using a 3 M LiClO and 0.1 M HClO wash at a flowrate of 0.4 ml
4
4
–1
min .
Concentration (mM) a
Sample description
D-Valine-ester L-Valine ester
0–5 ml feed effluent 18.5 31.8
5–9 ml feed effluent 14.8 23.0
9–14 ml feed effluent 9.8 15.0
14–19 ml feed effluent 7.9 6.4
a Analyzed by HPLC.
Table 8-6. Single column elution of a 3.9 g ChiraLig™ column using an H O elution at 0.2 ml min –1
2
following column loading (25 mMD-methyl ester valine versus 25 mML-methyl ester valine) and
washing.
Concentration (mM) a
Sample description
D-Valine-ester L-Valine ester
0–5 ml elution effluent 22.0 8.8
5–7 ml elution effluent 28.0 5.2
7–10 ml elution effluent 29.6 1.7
10–15.5 ml elution effluent 24.7 2.8
15.5–19.5 ml elution effluent 21.5 2.1
19.5–23.5 ml elution effluent 13.8 1.0
23.5–27.5 ml elution effluent 9.2 0.8
Σ mmol combined eluent 0.57 0.09
a Analyzed by HPLC.
lead column. This is important since the nonselective feed would partially contami-
nate the bound valine ester if not washed out prior to the elution. The wash is the
same as the feed matrix, so that minimal bound valine ester is lost. For purposes of
collecting the wash data, the wash in Table 8-5 was collected for analysis. However,
in the rest of the operation the wash volume is sent on to the trail column(s) so that
this volume is not lost to the separation.
Finally, the data in Table 8-6 show the elution of the lead column. The eluent is
–
H O. The driving force for the elution in this case is the lack of ClO present to act
2 4
as an anion in the binding of the ammonium perchlorate salt pair. The D-enantiomer
versus L-enantiomer ratio in the elution is slightly greater than 6:1, as expected by
the inherent selectivity of the ligand. For this separation system, LiClO is then
4
added back to the eluent and the eluent is sent on as load to the next purification
stage.
The other stages operate similarly. The α value of 6 6 allows for enantiomeric
purity of 6 97 % for two stages and 6 99.5 % for three stages. Hence, these results
demonstrate the applicability of the systems and technology to performing an enan-
tiomeric separation in a two-to-three-stage nonchromatographic bind/release rather
than chromatographic system. Full capacity of the ChiraLig TM resins are used in