Page 234 - Chiral Separation Techniques
P. 234
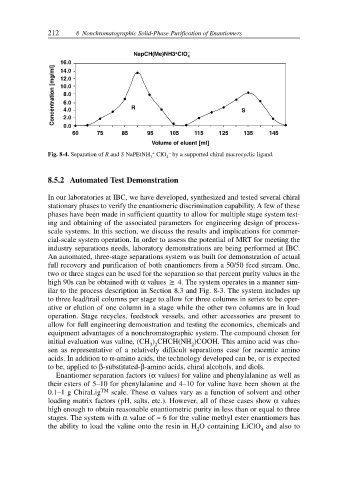
212 8 Nonchromatographic Solid-Phase Purification of Enantiomers
+
NapCH(Me)NH3 ClO –
4
16.0
Concentration [mg/ml] 10.0 R S
14.0
12.0
8.0
6.0
4.0
2.0
0.0
60 75 85 95 105 115 125 135 145
Volume of eluent [ml]
–
Fig. 8-4. Separation of R and S NaPEtNH + ClO by a supported chiral macrocyclic ligand.
3 4
8.5.2 Automated Test Demonstration
In our laboratories at IBC, we have developed, synthesized and tested several chiral
stationary phases to verify the enantiomeric discrimination capability. A few of these
phases have been made in sufficient quantity to allow for multiple stage system test-
ing and obtaining of the associated parameters for engineering design of process-
scale systems. In this section, we discuss the results and implications for commer-
cial-scale system operation. In order to assess the potential of MRT for meeting the
industry separations needs, laboratory demonstrations are being performed at IBC.
An automated, three-stage separations system was built for demonstration of actual
full recovery and purification of both enantiomers from a 50/50 feed stream. One,
two or three stages can be used for the separation so that percent purity values in the
high 90s can be obtained with α values 6 4. The system operates in a manner sim-
ilar to the process description in Section 8.3 and Fig. 8-3. The system includes up
to three lead/trail columns per stage to allow for three columns in series to be oper-
ative or elution of one column in a stage while the other two columns are in load
operation. Stage recycles, feedstock vessels, and other accessories are present to
allow for full engineering demonstration and testing the economics, chemicals and
equipment advantages of a nonchromatographic system. The compound chosen for
initial evaluation was valine, (CH ) CHCH(NH )COOH. This amino acid was cho-
3 2 2
sen as representative of a relatively difficult separations case for racemic amino
acids. In addition to α-amino acids, the technology developed can be, or is expected
to be, applied to β-substituted-β-amino acids, chiral alcohols, and diols.
Enantiomer separation factors (α values) for valine and phenylalanine as well as
their esters of 5–10 for phenylalanine and 4–10 for valine have been shown at the
0.1–1 g ChiraLig TM scale. These α values vary as a function of solvent and other
loading matrix factors (pH, salts, etc.). However, all of these cases show α values
high enough to obtain reasonable enantiometric purity in less than or equal to three
stages. The system with α value of ≈ 6 for the valine methyl ester enantiomers has
the ability to load the valine onto the resin in H O containing LiClO and also to
2 4