Page 255 - Chiral Separation Techniques
P. 255
9.4 Simulation Results 233
For nonlinear systems, however, the evaluation of the flow rates is not straight-
forward. Morbidelli and co-workers developed a complete design of the binary sep-
aration by SMB chromatography in the frame of Equilibrium Theory for various
adsorption equilibrium isotherms: the constant selectivity stoichiometric model [21,
22], the constant selectivity Langmuir adsorption isotherm [23], the variable selec-
tivity modified Langmuir isotherm [24], and the bi-Langmuir isotherm [25]. The
region for complete separation was defined in terms of the flow rate ratios in the four
sections of the equivalent TMB unit:
Qt − ε V
**
m = j c (33)
j
(1 − V ) ε c
which are related to the γ ratios used in this work by:
j
γ = 1 − ε m (34)
j ε j
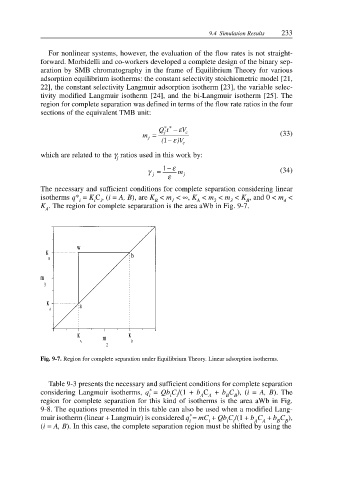
The necessary and sufficient conditions for complete separation considering linear
isotherms q* = K C ,(i = A, B), are K < m < ∞, K < m < m < K , and 0 < m <
i i i B 1 A 2 3 B 4
K . The region for complete separaration is the area aWb in Fig. 9-7.
A
Fig. 9-7. Region for complete separation under Equilibrium Theory. Linear adsorption isotherms.
Table 9-3 presents the necessary and sufficient conditions for complete separation
*
considering Langmuir isotherms, q = Qb C /(1 + b C + b C ), (i = A, B). The
i i i A A B B
region for complete separation for this kind of isotherms is the area aWb in Fig.
9-8. The equations presented in this table can also be used when a modified Lang-
*
muir isotherm (linear + Langmuir) is considered q = mC + Qb C /(1 + b C + b C ),
i i i i A A B B
(i = A, B). In this case, the complete separation region must be shifted by using the