Page 75 - Chiral Separation Techniques
P. 75
2.4 Optimization 51
2.4.3.2 Effect of Aqueous Buffer on Chiral Separations
For most free amino acids and small peptides, a mixture of alcohol with water is a
typical mobile phase composition in the reversed-phase mode for glycopeptide
CSPs. For some bifunctional amino acids and most other compounds, however,
aqueous buffer is usually necessary to enhance resolution. The types of buffers dic-
tate the retention, efficiency and – to a lesser effect – selectivity of analytes. Tri-
ethylammonium acetate and ammonium nitrate are the most effective buffer sys-
tems, while sodium citrate is also effective for the separation of profens on van-
comycin CSP, and ammonium acetate is the most appropriate for LC/MS
applications.
Buffer pH is the most important parameter in chiral selectivity on all glycopep-
tide CSPs. The stability of the analyte–CSP complex depends on the degree of inter-
action between both entities. Therefore, retention and selectivity of molecules pos-
sessing ionizable (acidic or basic) or even neutral functional groups can be affected
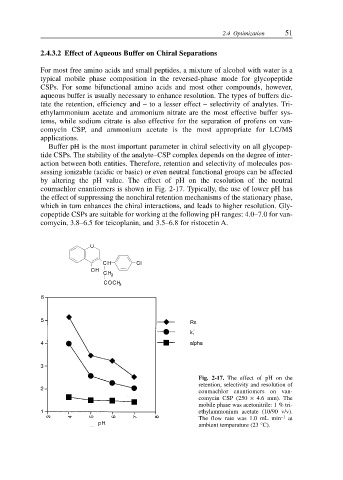
by altering the pH value. The effect of pH on the resolution of the neutral
coumachlor enantiomers is shown in Fig. 2-17. Typically, the use of lower pH has
the effect of suppressing the nonchiral retention mechanisms of the stationary phase,
which in turn enhances the chiral interactions, and leads to higher resolution. Gly-
copeptide CSPs are suitable for working at the following pH ranges: 4.0–7.0 for van-
comycin, 3.8–6.5 for teicoplanin, and 3.5–6.8 for ristocetin A.
Fig. 2-17. The effect of pH on the
retention, selectivity and resolution of
coumachlor enantiomers on van-
comycin CSP (250 × 4.6 mm). The
mobile phase was acetonitrile: 1 % tri-
ethylammonium acetate (10/90 v/v).
The flow rate was 1.0 mL min –1 at
ambient temperature (23 °C).