Page 70 - Chiral Separation Techniques
P. 70
46 2 Method Development and Optimization of Enantiomeric Separations Using …
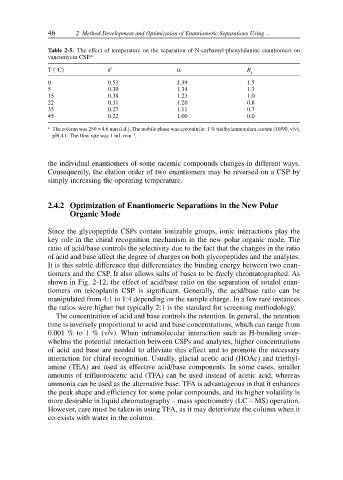
Table 2-5. The effect of temperature on the separation of N-carbamyl-phenylalanine enantiomers on
vancomycin CSP. a
T (°C) k α R
s
0 0.51 1.39 1.5
5 0.39 1.34 1.3
15 0.38 1.23 1.0
22 0.31 1.20 0.8
35 0.27 1.11 0.7
45 0.22 1.00 0.0
a The column was 250 × 4.6 mm (i.d.). The mobile phase was acetonitrile: 1 % triethylammonium acetate (10/90, v/v),
–1
pH 4.1. The flow rate was 1 mL min .
the individual enantiomers of some racemic compounds changes in different ways.
Consequently, the elution order of two enantiomers may be reversed on a CSP by
simply increasing the operating temperature.
2.4.2 Optimization of Enantiomeric Separations in the New Polar
Organic Mode
Since the glycopeptide CSPs contain ionizable groups, ionic interactions play the
key role in the chiral recognition mechanism in the new polar organic mode. The
ratio of acid/base controls the selectivity due to the fact that the changes in the ratio
of acid and base affect the degree of charges on both glycopeptides and the analytes.
It is this subtle difference that differentiates the binding energy between two enan-
tiomers and the CSP. It also allows salts of bases to be freely chromatographed. As
shown in Fig. 2-12, the effect of acid/base ratio on the separation of sotalol enan-
tiomers on teicoplanin CSP is significant. Generally, the acid/base ratio can be
manipulated from 4:1 to 1:4 depending on the sample charge. In a few rare instances
the ratios were higher but typically 2:1 is the standard for screening methodology.
The concentration of acid and base controls the retention. In general, the retention
time is inversely proportional to acid and base concentrations, which can range from
0.001 % to 1 % (v/v). When intramolecular interaction such as H-bonding over-
whelms the potential interaction between CSPs and analytes, higher concentrations
of acid and base are needed to alleviate this effect and to promote the necessary
interaction for chiral recognition. Usually, glacial acetic acid (HOAc) and triethyl-
amine (TEA) are used as effective acid/base components. In some cases, smaller
amounts of trifluoroacetic acid (TFA) can be used instead of acetic acid, whereas
ammonia can be used as the alternative base. TFA is advantageous in that it enhances
the peak shape and efficiency for some polar compounds, and its higher volatility is
more desirable in liquid chromatography – mass spectrometry (LC – MS) operation.
However, care must be taken in using TFA, as it may deteriorate the column when it
co-exists with water in the column.