Page 65 - Chiral Separation Techniques
P. 65
2.3 Method Development with Glycopeptide CSPs 41
A B C
D E F
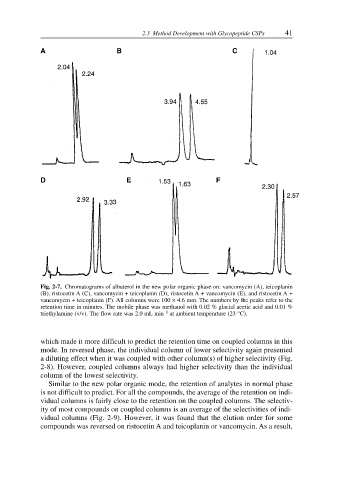
Fig. 2-7. Chromatograms of albuterol in the new polar organic phase on: vancomycin (A), teicoplanin
(B), ristocetin A (C), vancomycin + teicoplanin (D), ristocetin A + vancomycin (E), and ristocetin A +
vancomycin + teicoplanin (F). All columns were 100 × 4.6 mm. The numbers by the peaks refer to the
retention time in minutes. The mobile phase was methanol with 0.02 % glacial acetic acid and 0.01 %
–1
triethylamine (v/v). The flow rate was 2.0 mL min at ambient temperature (23 °C).
which made it more difficult to predict the retention time on coupled columns in this
mode. In reversed phase, the individual column of lower selectivity again presented
a diluting effect when it was coupled with other column(s) of higher selectivity (Fig.
2-8). However, coupled columns always had higher selectivity than the individual
column of the lowest selectivity.
Similar to the new polar organic mode, the retention of analytes in normal phase
is not difficult to predict. For all the compounds, the average of the retention on indi-
vidual columns is fairly close to the retention on the coupled columns. The selectiv-
ity of most compounds on coupled columns is an average of the selectivities of indi-
vidual columns (Fig. 2-9). However, it was found that the elution order for some
compounds was reversed on ristocetin A and teicoplanin or vancomycin. As a result,