Page 69 - Chiral Separation Techniques
P. 69
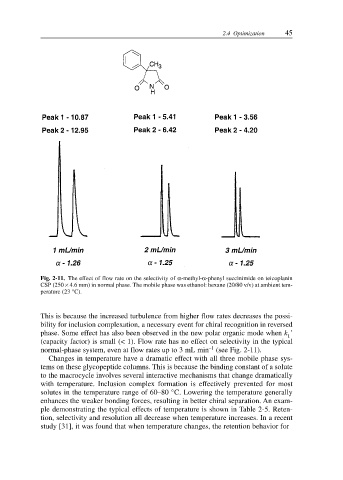
2.4 Optimization 45
Fig. 2-11. The effect of flow rate on the selectivity of α-methyl-α-phenyl succinimide on teicoplanin
CSP (250 × 4.6 mm) in normal phase. The mobile phase was ethanol: hexane (20/80 v/v) at ambient tem-
perature (23 °C).
This is because the increased turbulence from higher flow rates decreases the possi-
bility for inclusion complexation, a necessary event for chiral recognition in reversed
phase. Some effect has also been observed in the new polar organic mode when k
1
(capacity factor) is small (< 1). Flow rate has no effect on selectivity in the typical
–1
normal-phase system, even at flow rates up to 3 mL min (see Fig. 2-11).
Changes in temperature have a dramatic effect with all three mobile phase sys-
tems on these glycopeptide columns. This is because the binding constant of a solute
to the macrocycle involves several interactive mechanisms that change dramatically
with temperature. Inclusion complex formation is effectively prevented for most
solutes in the temperature range of 60–80 °C. Lowering the temperature generally
enhances the weaker bonding forces, resulting in better chiral separation. An exam-
ple demonstrating the typical effects of temperature is shown in Table 2-5. Reten-
tion, selectivity and resolution all decrease when temperature increases. In a recent
study [31], it was found that when temperature changes, the retention behavior for