Page 73 - Chiral Separation Techniques
P. 73
2.4 Optimization 49
Table 2-6. The effects of organic modifier on the separation of amino acids on teicoplanin CSP. a
Methanol Ethanol Isopropanol b
Compound
k α R k α R k α Rs
1 s 1 s 1
o-Thiophenylglycine 1.63 2.4 4.7 0.57 4.9 5.4 0.80 4.9 10.1
3-(1-Naphthyl) alanine 2.79 1.3 1.8 1.0 1.6 1.3 1.04 1.8 3.8
Pipecolic acid 2.25 1.6 1.7 1.12 2.0 1.9 1.32 2.2 4.0
Threonine 1.40 1.1 1.0 0.99 1.5 1.6 0.62 1.3 1.6
a These data were generated with a 250 × 4.6 mm teicoplanin column. The mobile phase consisted of alcohol: water
–1
(60/40, v/v), and the flow rate was 1 mL min at ambient temperature (23 °C).
b Flow rate was 0.5 mL min due to the viscosity of the propanol: water mobile phase that produced a high back-
–1
pressure.
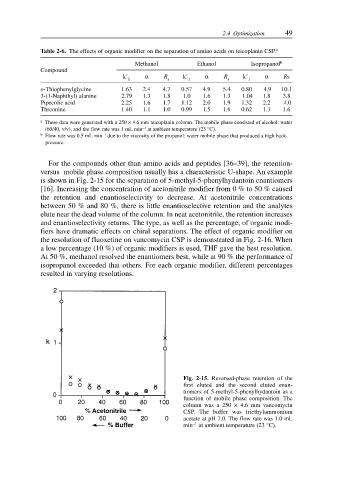
For the compounds other than amino acids and peptides [36–39], the retention-
versus mobile phase composition usually has a characteristic U-shape. An example
is shown in Fig. 2-15 for the separation of 5-methyl-5-phenylhydantoin enantiomers
[16]. Increasing the concentration of acetonitrile modifier from 0 % to 50 % caused
the retention and enantioselectivity to decrease. At acetonitrile concentrations
between 50 % and 80 %, there is little enantioselective retention and the analytes
elute near the dead volume of the column. In neat acetonitrile, the retention increases
and enantioselectivity returns. The type, as well as the percentage, of organic modi-
fiers have dramatic effects on chiral separations. The effect of organic modifier on
the resolution of fluoxetine on vancomycin CSP is demonstrated in Fig. 2-16. When
a low percentage (10 %) of organic modifiers is used, THF gave the best resolution.
At 50 %, methanol resolved the enantiomers best, while at 90 % the performance of
isopropanol exceeded that others. For each organic modifier, different percentages
resulted in varying resolutions.
Fig. 2-15. Reversed-phase retention of the
first eluted and the second eluted enan-
tiomers of 5-methyl-5-phenylhydantoin as a
function of mobile phase composition. The
column was a 250 × 4.6 mm vancomycin
CSP. The buffer was triethylammonium
acetate at pH 7.0. The flow rate was 1.0 mL
–1
min at ambient temperature (23 °C).