Page 217 - Computational Modeling in Biomedical Engineering and Medical Physics
P. 217
206 Computational Modeling in Biomedical Engineering and Medical Physics
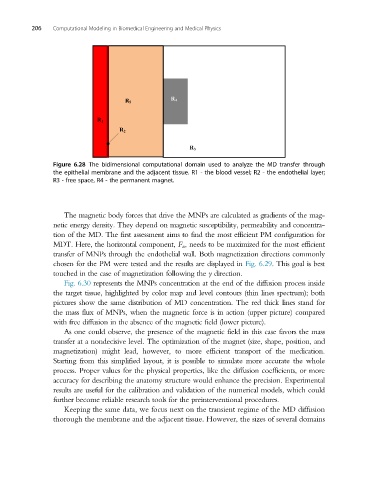
R 4
R 5
R 1
R 2
R 3
Figure 6.28 The bidimensional computational domain used to analyze the MD transfer through
the epithelial membrane and the adjacent tissue. R1 - the blood vessel; R2 - the endothelial layer;
R3 - free space, R4 - the permanent magnet.
The magnetic body forces that drive the MNPs are calculated as gradients of the mag-
netic energy density. They depend on magnetic susceptibility, permeability and concentra-
tion of the MD. The first assessment aims to find the most efficient PM configuration for
MDT. Here, the horizontal component, F x ,needs to be maximizedfor the most efficient
transfer of MNPs through the endothelial wall. Both magnetization directions commonly
chosen for the PM were tested and the results are displayed in Fig. 6.29. This goal is best
touched in the case of magnetization following the y direction.
Fig. 6.30 represents the MNPs concentration at the end of the diffusion process inside
the target tissue, highlighted by color map and level contours (thin lines spectrum); both
pictures show the same distribution of MD concentration. The red thick lines stand for
the mass flux of MNPs, when the magnetic force is in action (upper picture) compared
with free diffusion in the absence of the magnetic field (lower picture).
As one could observe, the presence of the magnetic field in this case favors the mass
transfer at a nondecisive level. The optimization of the magnet (size, shape, position, and
magnetization) might lead, however, to more efficient transport of the medication.
Starting from this simplified layout, it is possible to simulate more accurate the whole
process. Proper values for the physical properties, like the diffusion coefficients, or more
accuracy for describing the anatomy structure would enhance the precision. Experimental
results are useful for the calibration and validation of the numerical models, which could
further become reliable research tools for the preinterventional procedures.
Keeping the same data, we focus next on the transient regime of the MD diffusion
thorough the membrane and the adjacent tissue. However, the sizes of several domains