Page 103 - Concise Encyclopedia of Robotics
P. 103
Electrochemical Power
Primary and secondary cells
Some cells, once their chemical energy has all been changed to electricity
and used up, must be discarded. These are primary cells. Other kinds of
cells,like the lead–acid unit described above,can get their chemical energy
back again by means of recharging. Such a component is a secondary cell.
Primary cells contain a dry electrolyte paste along with metal electrodes.
They go by names such as dry cell, zinc–carbon cell, or alkaline cell. These
cells are commonly found in supermarkets and other stores. Some
secondary cells can also be found at the consumer level. Nickel–cadmium
(Ni–Cd or NICAD) cells are one common type. These cost more than
ordinary dry cells, and a charging unit also costs a few dollars. However,
these rechargeable cells can be used hundreds of times, and can pay for
themselves and the charger several times over.
An automotive battery is made from secondary cells connected in series.
These cells recharge from an alternator or from an outside charging unit.
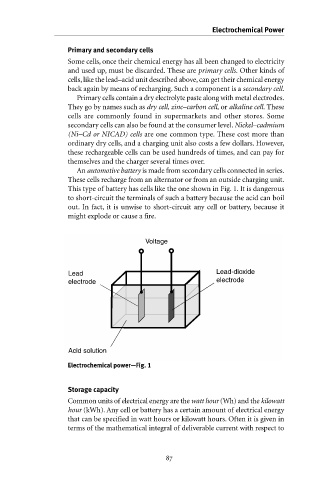
This type of battery has cells like the one shown in Fig. 1. It is dangerous
to short-circuit the terminals of such a battery because the acid can boil
out. In fact, it is unwise to short-circuit any cell or battery, because it
might explode or cause a fire.
Voltage
Lead Lead-dioxide
electrode electrode
Acid solution
Electrochemical power—Fig. 1
Storage capacity
Common units of electrical energy are the watt hour (Wh) and the kilowatt
hour (kWh). Any cell or battery has a certain amount of electrical energy
that can be specified in watt hours or kilowatt hours. Often it is given in
terms of the mathematical integral of deliverable current with respect to