Page 105 - Concise Encyclopedia of Robotics
P. 105
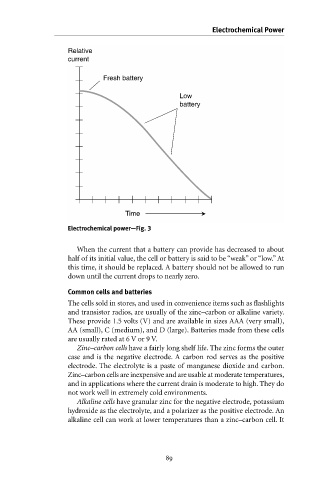
Relative
current
Fresh battery
battery
Time Low Electrochemical Power
Electrochemical power—Fig. 3
When the current that a battery can provide has decreased to about
half of its initial value, the cell or battery is said to be “weak” or “low.” At
this time, it should be replaced. A battery should not be allowed to run
down until the current drops to nearly zero.
Common cells and batteries
The cells sold in stores, and used in convenience items such as flashlights
and transistor radios, are usually of the zinc–carbon or alkaline variety.
These provide 1.5 volts (V) and are available in sizes AAA (very small),
AA (small), C (medium), and D (large). Batteries made from these cells
are usually rated at 6 V or 9 V.
Zinc–carbon cells have a fairly long shelf life. The zinc forms the outer
case and is the negative electrode. A carbon rod serves as the positive
electrode. The electrolyte is a paste of manganese dioxide and carbon.
Zinc–carbon cells are inexpensive and are usable at moderate temperatures,
and in applications where the current drain is moderate to high. They do
not work well in extremely cold environments.
Alkaline cells have granular zinc for the negative electrode, potassium
hydroxide as the electrolyte, and a polarizer as the positive electrode. An
alkaline cell can work at lower temperatures than a zinc–carbon cell. It