Page 103 - Control Theory in Biomedical Engineering
P. 103
90 Control theory in biomedical engineering
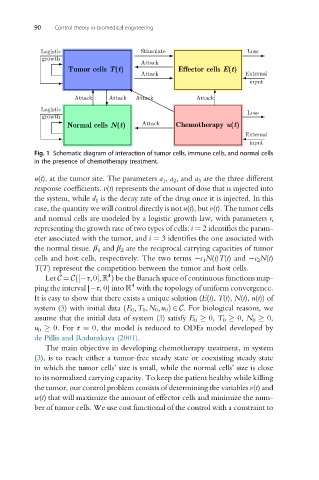
Stimulate
Logistic Loss
growth Attack
Tumor cells T(t) Effector cells E(t)
Attack External
input
Attack Attack Attack Attack
Logistic
Loss
growth
Normal cells N(t) Attack Chemotherapy u(t)
External
input
Fig. 1 Schematic diagram of interaction of tumor cells, immune cells, and normal cells
in the presence of chemotherapy treatment.
u(t), at the tumor site. The parameters a 1 , a 2 , and a 3 are the three different
response coefficients. v(t) represents the amount of dose that is injected into
the system, while d 1 is the decay rate of the drug once it is injected. In this
case, the quantity we will control directly is not u(t), but v(t). The tumor cells
and normal cells are modeled by a logistic growth law, with parameters r i
representing the growth rate of two types of cells: i ¼ 2 identifies the param-
eter associated with the tumor, and i ¼ 3 identifies the one associated with
the normal tissue. β 1 and β 2 are the reciprocal carrying capacities of tumor
cells and host cells, respectively. The two terms c 1 N(t)T(t) and c 2 N(t)
T(T) represent the competition between the tumor and host cells.
4
Let C ¼ Cð½ τ,0, Þ be the Banach space of continuous functions map-
4
ping the interval [ τ, 0] into with the topology of uniform convergence.
It is easy to show that there exists a unique solution (E(t), T(t), N(t), u(t)) of
system (3) with initial data ðE 0 ,T 0 ,N 0 ,u 0 Þ C. For biological reasons, we
assume that the initial data of system (3) satisfy E 0 0, T 0 0, N 0 0,
u 0 0. For τ ¼ 0, the model is reduced to ODEs model developed by
de Pillis and Radunskaya (2001).
The main objective in developing chemotherapy treatment, in system
(3), is to reach either a tumor-free steady state or coexisting steady state
in which the tumor cells’ size is small, while the normal cells’ size is close
to its normalized carrying capacity. To keep the patient healthy while killing
the tumor, our control problem consists of determining the variables v(t) and
w(t) that will maximize the amount of effector cells and minimize the num-
ber of tumor cells. We use cost functional of the control with a constraint to