Page 334 - Control Theory in Biomedical Engineering
P. 334
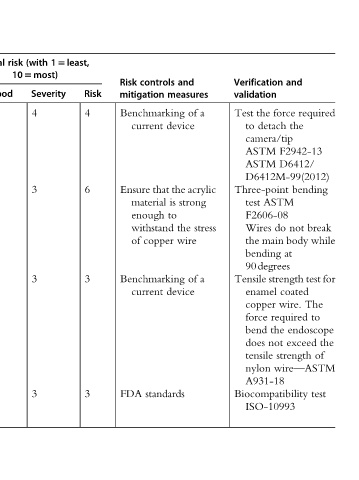
required bending break while for test The to endoscope the of test
and force the F2942-13 D6412/ D6412M-99(2012) ASTM not do body at strength coated wire. required the exceed strength wire—ASTM
Verification validation the Test detach to camera/tip ASTM ASTM Three-point test F2606-08 Wires main the bending 90degrees Tensile enamel copper force bend not does tensile nylon A931-18 Biocompatibility ISO-10993
and measures a of device acrylic the strong is to stress the wire a of device
controls mitigation Benchmarking current that material enough withstand copper Benchmarking current standards
Risk Ensure of FDA
Risk 4 6 3 3
15least,
(with 105most) Severity 4 3 3 3
risk
Initial Likelihood 2 1 1 1
tip a in a in (unable the
flexible is trapped in a patient’s of endoscope trapped patient’s of endoscope trapped patient’s retract endoscope) body response, inflammation
Harm The body Part body Part body to Foreign
failure bending tip principal the tensile the of coated wire biocompatibility material
of the Exceeding of material Exceeding strength enamel copper Inadequate the
Cause Repeated of stress of
analysis tip main
mode mode of endoscope endoscopy of design breakage endoscopy Biocompatibility materials (surface
Failure Failure Breakage during Breakage body Wire during of used