Page 166 - Corrosion Engineering Principles and Practice
P. 166
140 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 141
Sealing of Anodic Coatings
To achieve the maximum protective qualities and corrosion resistance
required for finished articles, the anodic oxide must be sealed after it
is formed and/or colored. This produces a hydrated oxide layer with
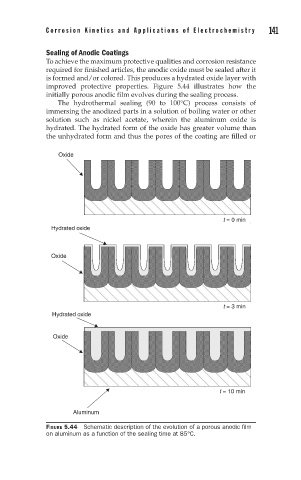
improved protective properties. Figure 5.44 illustrates how the
initially porous anodic film evolves during the sealing process.
The hydrothermal sealing (90 to 100°C) process consists of
immersing the anodized parts in a solution of boiling water or other
solution such as nickel acetate, wherein the aluminum oxide is
hydrated. The hydrated form of the oxide has greater volume than
the unhydrated form and thus the pores of the coating are filled or
Oxide
t = 0 min
Hydrated oxide
Oxide
t = 3 min
Hydrated oxide
Oxide
t = 10 min
Aluminum
FIGURE 5.44 Schematic description of the evolution of a porous anodic film
on aluminum as a function of the sealing time at 85°C.