Page 161 - Corrosion Engineering Principles and Practice
P. 161
136 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 137
protective anodic film can be damaged and even break down
completely. It follows that although a high current density may be
required to cause passivation (> i ), only a small current density is
cc
required to maintain it, and that in the passive region the corrosion
rate corresponds to the passive current density (i ).
p
Anodic protection possesses unique features. For example, the
applied current is usually equal to the corrosion rate of the protected
system. Thus, anodic protection not only protects but also offers a
direct means for monitoring the corrosion rate of a system. The
main advantages of anodic protection are (1) low current
requirements; (2) large reductions in corrosion rate (typically
10,000-fold or more); and (3) applicability to certain strong, hot
acids and other highly corrosive media. It is important to emphasize
that anodic protection can only be applied to metals and alloys
possessing active-passive characteristics such as titanium, stainless
steels, steel, and nickel-base alloys.
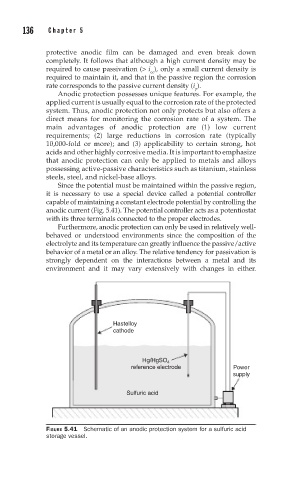
Since the potential must be maintained within the passive region,
it is necessary to use a special device called a potential controller
capable of maintaining a constant electrode potential by controlling the
anodic current (Fig. 5.41). The potential controller acts as a potentiostat
with its three terminals connected to the proper electrodes.
Furthermore, anodic protection can only be used in relatively well-
behaved or understood environments since the composition of the
electrolyte and its temperature can greatly influence the passive/active
behavior of a metal or an alloy. The relative tendency for passivation is
strongly dependent on the interactions between a metal and its
environment and it may vary extensively with changes in either.
Hastelloy
cathode
Hg/HgSO
4
reference electrode Power
supply
Sulfuric acid
FIGURE 5.41 Schematic of an anodic protection system for a sulfuric acid
storage vessel.