Page 160 - Corrosion Engineering Principles and Practice
P. 160
134 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 135
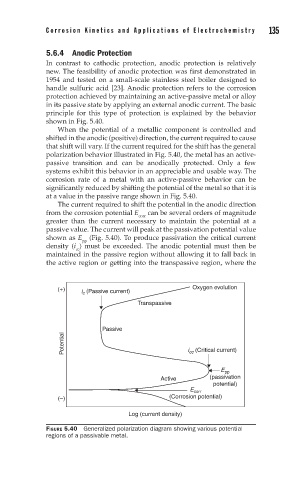
5.6.4 Anodic Protection
In contrast to cathodic protection, anodic protection is relatively
new. The feasibility of anodic protection was first demonstrated in
1954 and tested on a small-scale stainless steel boiler designed to
handle sulfuric acid [23]. Anodic protection refers to the corrosion
protection achieved by maintaining an active-passive metal or alloy
in its passive state by applying an external anodic current. The basic
principle for this type of protection is explained by the behavior
shown in Fig. 5.40.
When the potential of a metallic component is controlled and
shifted in the anodic (positive) direction, the current required to cause
that shift will vary. If the current required for the shift has the general
polarization behavior illustrated in Fig. 5.40, the metal has an active-
passive transition and can be anodically protected. Only a few
systems exhibit this behavior in an appreciable and usable way. The
corrosion rate of a metal with an active-passive behavior can be
significantly reduced by shifting the potential of the metal so that it is
at a value in the passive range shown in Fig. 5.40.
The current required to shift the potential in the anodic direction
from the corrosion potential E corr can be several orders of magnitude
greater than the current necessary to maintain the potential at a
passive value. The current will peak at the passivation potential value
shown as E (Fig. 5.40). To produce passivation the critical current
pp
density (i ) must be exceeded. The anodic potential must then be
cc
maintained in the passive region without allowing it to fall back in
the active region or getting into the transpassive region, where the
(+) i (Passive current) Oxygen evolution
c
Transpassive
Passive
Potential i (Critical current)
cc
E pp
Active (passivation
potential)
E corr
(–) (Corrosion potential)
Log (current density)
FIGURE 5.40 Generalized polarization diagram showing various potential
regions of a passivable metal.