Page 164 - Corrosion Engineering Principles and Practice
P. 164
138 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 139
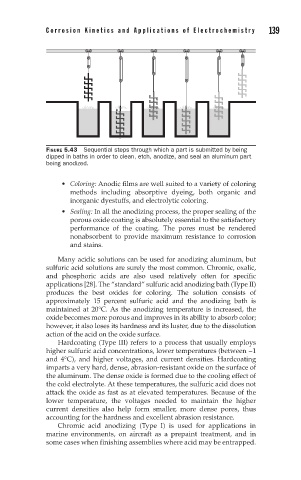
FIGURE 5.43 Sequential steps through which a part is submitted by being
dipped in baths in order to clean, etch, anodize, and seal an aluminum part
being anodized.
• Coloring: Anodic films are well suited to a variety of coloring
methods including absorptive dyeing, both organic and
inorganic dyestuffs, and electrolytic coloring.
• Sealing: In all the anodizing process, the proper sealing of the
porous oxide coating is absolutely essential to the satisfactory
performance of the coating. The pores must be rendered
nonabsorbent to provide maximum resistance to corrosion
and stains.
Many acidic solutions can be used for anodizing aluminum, but
sulfuric acid solutions are surely the most common. Chromic, oxalic,
and phosphoric acids are also used relatively often for specific
applications [28]. The “standard” sulfuric acid anodizing bath (Type II)
produces the best oxides for coloring. The solution consists of
approximately 15 percent sulfuric acid and the anodizing bath is
maintained at 20°C. As the anodizing temperature is increased, the
oxide becomes more porous and improves in its ability to absorb color;
however, it also loses its hardness and its luster, due to the dissolution
action of the acid on the oxide surface.
Hardcoating (Type III) refers to a process that usually employs
higher sulfuric acid concentrations, lower temperatures (between −1
and 4°C), and higher voltages, and current densities. Hardcoating
imparts a very hard, dense, abrasion-resistant oxide on the surface of
the aluminum. The dense oxide is formed due to the cooling effect of
the cold electrolyte. At these temperatures, the sulfuric acid does not
attack the oxide as fast as at elevated temperatures. Because of the
lower temperature, the voltages needed to maintain the higher
current densities also help form smaller, more dense pores, thus
accounting for the hardness and excellent abrasion resistance.
Chromic acid anodizing (Type I) is used for applications in
marine environments, on aircraft as a prepaint treatment, and in
some cases when finishing assemblies where acid may be entrapped.