Page 162 - Corrosion Engineering Principles and Practice
P. 162
136 C h a p t e r 5 C o r r o s i o n K i n e t i c s a n d A p p l i c a t i o n s o f E l e c t r o c h e m i s t r y 137
1250
1050 No Sensitization 1000 h
Potential (mV vs. SHE) 650 1 h
850
0.3 h
450
250
50
–150
–2 –1 0 1 2 3 4 5
–2
Log current density (µA cm )
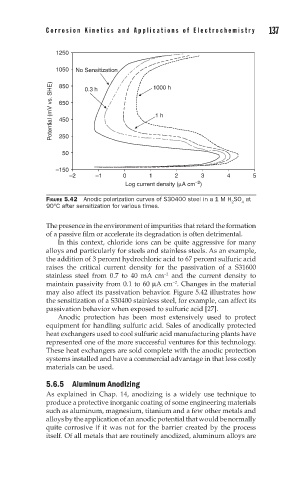
FIGURE 5.42 Anodic polarization curves of S30400 steel in a 1 M H SO at
2
4
90°C after sensitization for various times.
The presence in the environment of impurities that retard the formation
of a passive film or accelerate its degradation is often detrimental.
In this context, chloride ions can be quite aggressive for many
alloys and particularly for steels and stainless steels. As an example,
the addition of 3 percent hydrochloric acid to 67 percent sulfuric acid
raises the critical current density for the passivation of a S31600
−2
stainless steel from 0.7 to 40 mA cm and the current density to
maintain passivity from 0.1 to 60 µA cm . Changes in the material
−2
may also affect its passivation behavior. Figure 5.42 illustrates how
the sensitization of a S30400 stainless steel, for example, can affect its
passivation behavior when exposed to sulfuric acid [27].
Anodic protection has been most extensively used to protect
equipment for handling sulfuric acid. Sales of anodically protected
heat exchangers used to cool sulfuric acid manufacturing plants have
represented one of the more successful ventures for this technology.
These heat exchangers are sold complete with the anodic protection
systems installed and have a commercial advantage in that less costly
materials can be used.
5.6.5 Aluminum Anodizing
As explained in Chap. 14, anodizing is a widely use technique to
produce a protective inorganic coating of some engineering materials
such as aluminum, magnesium, titanium and a few other metals and
alloys by the application of an anodic potential that would be normally
quite corrosive if it was not for the barrier created by the process
itself. Of all metals that are routinely anodized, aluminum alloys are