Page 225 - Corrosion Engineering Principles and Practice
P. 225
200 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 201
where s is mean stress
m
a is depth of defect
C is geometric constant
K is critical stress intensity at which catastrophic fracture
I c
occurs
SCC is a mechanical–chemical process leading to the cracking of
certain alloys at stresses below their tensile strengths. A susceptible
alloy, the proper chemical environment, plus an enduring tensile
stress are required. It is likely that there are no alloy systems which
are completely immune to SCC in all environments. Usually, there is
an induction period, during which cracking nucleates at a microscopic
level. This period of latency may be quite long (e.g., many months or
years) before progressing to the propagation stage.
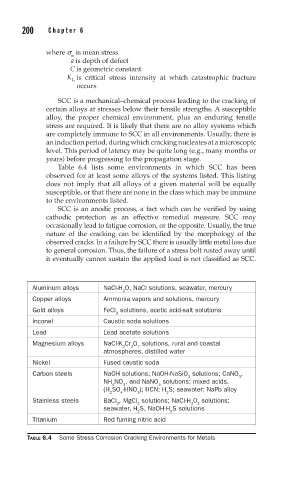
Table 6.4 lists some environments in which SCC has been
observed for at least some alloys of the systems listed. This listing
does not imply that all alloys of a given material will be equally
susceptible, or that there are none in the class which may be immune
to the environments listed.
SCC is an anodic process, a fact which can be verified by using
cathodic protection as an effective remedial measure. SCC may
occasionally lead to fatigue corrosion, or the opposite. Usually, the true
nature of the cracking can be identified by the morphology of the
observed cracks. In a failure by SCC there is usually little metal loss due
to general corrosion. Thus, the failure of a stress bolt rusted away until
it eventually cannot sustain the applied load is not classified as SCC.
Aluminum alloys NaCl-H O, NaCl solutions, seawater, mercury
2
Copper alloys Ammonia vapors and solutions, mercury
Gold alloys FeCl solutions, acetic acid-salt solutions
3
Inconel Caustic soda solutions
Lead Lead acetate solutions
Magnesium alloys NaCl-K Cr O solutions, rural and coastal
7
2
2
atmospheres, distilled water
Nickel Fused caustic soda
Carbon steels NaOH solutions; NaOH-NaSiO solutions; CaNO ,
3
3
NH NO , and NaNO solutions; mixed acids,
3
3
4
(H SO -HNO ); HCN; H S; seawater; NaPb alloy
2 4 3 2
Stainless steels BaCl , MgCl solutions; NaCl-H 0 solutions;
2
2
2
2
seawater, H S, NaOH-H S solutions
2 2
Titanium Red fuming nitric acid
TABLE 6.4 Some Stress Corrosion Cracking Environments for Metals