Page 275 - Corrosion Engineering Principles and Practice
P. 275
248 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 249
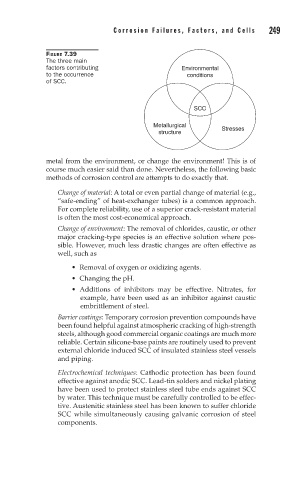
FIGURE 7.39
The three main
factors contributing Environmental
to the occurrence conditions
of SCC.
SCC
Metallurgical Stresses
structure
metal from the environment, or change the environment! This is of
course much easier said than done. Nevertheless, the following basic
methods of corrosion control are attempts to do exactly that.
Change of material: A total or even partial change of material (e.g.,
“safe-ending” of heat-exchanger tubes) is a common approach.
For complete reliability, use of a superior crack-resistant material
is often the most cost-economical approach.
Change of environment: The removal of chlorides, caustic, or other
major cracking-type species is an effective solution where pos-
sible. However, much less drastic changes are often effective as
well, such as
• Removal of oxygen or oxidizing agents.
• Changing the pH.
• Additions of inhibitors may be effective. Nitrates, for
example, have been used as an inhibitor against caustic
embrittlement of steel.
Barrier coatings: Temporary corrosion prevention compounds have
been found helpful against atmospheric cracking of high-strength
steels, although good commercial organic coatings are much more
reliable. Certain silicone-base paints are routinely used to prevent
external chloride induced SCC of insulated stainless steel vessels
and piping.
Electrochemical techniques: Cathodic protection has been found
effective against anodic SCC. Lead-tin solders and nickel plating
have been used to protect stainless steel tube ends against SCC
by water. This technique must be carefully controlled to be effec-
tive. Austenitic stainless steel has been known to suffer chloride
SCC while simultaneously causing galvanic corrosion of steel
components.