Page 278 - Corrosion Engineering Principles and Practice
P. 278
252 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 253
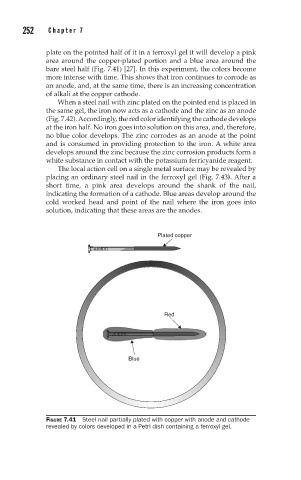
plate on the pointed half of it in a ferroxyl gel it will develop a pink
area around the copper-plated portion and a blue area around the
bare steel half (Fig. 7.41) [27]. In this experiment, the colors become
more intense with time. This shows that iron continues to corrode as
an anode, and, at the same time, there is an increasing concentration
of alkali at the copper cathode.
When a steel nail with zinc plated on the pointed end is placed in
the same gel, the iron now acts as a cathode and the zinc as an anode
(Fig. 7.42). Accordingly, the red color identifying the cathode develops
at the iron half. No iron goes into solution on this area, and, therefore,
no blue color develops. The zinc corrodes as an anode at the point
and is consumed in providing protection to the iron. A white area
develops around the zinc because the zinc corrosion products form a
white substance in contact with the potassium ferricyanide reagent.
The local action cell on a single metal surface may be revealed by
placing an ordinary steel nail in the ferroxyl gel (Fig. 7.43). After a
short time, a pink area develops around the shank of the nail,
indicating the formation of a cathode. Blue areas develop around the
cold worked head and point of the nail where the iron goes into
solution, indicating that these areas are the anodes.
Plated copper
Red
Blue
FIGURE 7.41 Steel nail partially plated with copper with anode and cathode
revealed by colors developed in a Petri dish containing a ferroxyl gel.