Page 277 - Corrosion Engineering Principles and Practice
P. 277
250 C h a p t e r 7 C o r r o s i o n F a i l u r e s , F a c t o r s , a n d C e l l s 251
ions or the reduction of oxygen. Phenolphthalein is a well-known
indicator which develops a red color when the concentration of
hydroxyl ions is increased. Therefore, if phenolphthalein turns red as
corrosion proceeds, it will detect the existence of a cathode and reveal
where it is [27].
Similarly, potassium ferricyanide is a reagent which produces, as
described in Eq. (7.3), a blue color by reaction with ferrous ions (Fe II)
as they form at the anodic areas when iron corrodes. The appearance
of this blue color, therefore, demonstrates the existence and location
of anodes on iron.
[
(
3Fe 2+ + 4 Fe CN) ] 3− ) → Fe Fe CN) ] ⋅ 4H O ( Prussian blue) + 6CN −
(
[
6 yellow(
4
2 2
6 3
(7.3)
Fe 3 3+ Fe 3+ Fe 2+
The combination of these two reagents in a gelling agent to which
sodium chloride is added is known as a ferroxyl solution*. Cushman
and Gardner made ample use of this medium to reveal local corrosion
cells and supported their theories with many pictures in their 1910
textbook.
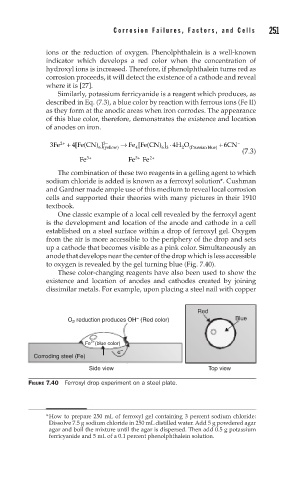
One classic example of a local cell revealed by the ferroxyl agent
is the development and location of the anode and cathode in a cell
established on a steel surface within a drop of ferroxyl gel. Oxygen
from the air is more accessible to the periphery of the drop and sets
up a cathode that becomes visible as a pink color. Simultaneously an
anode that develops near the center of the drop which is less accessible
to oxygen is revealed by the gel turning blue (Fig. 7.40).
These color-changing reagents have also been used to show the
existence and location of anodes and cathodes created by joining
dissimilar metals. For example, upon placing a steel nail with copper
Red
–
O reduction produces OH (Red color) Blue
2
2+
Fe (blue color)
e –
Corroding steel (Fe)
Side view Top view
FIGURE 7.40 Ferroxyl drop experiment on a steel plate.
* How to prepare 250 mL of ferroxyl gel containing 3 percent sodium chloride:
Dissolve 7.5 g sodium chloride in 250 mL distilled water. Add 5 g powdered agar
agar and boil the mixture until the agar is dispersed. Then add 0.5 g potassium
ferricyanide and 5 mL of a 0.1 percent phenolphthalein solution.