Page 38 - Corrosion Engineering Principles and Practice
P. 38
20 C h a p t e r 2 C o r r o s i o n B a s i c s 21
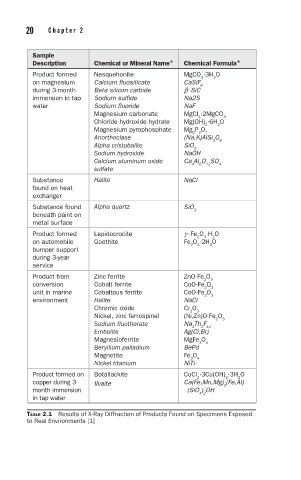
Sample
Description Chemical or Mineral Name* Chemical Formula*
Product formed Nesquehonite MgCO ·3H O
2
3
on magnesium Calcium fluosilicate CaSiF
6
during 3-month Beta silicon carbide b -SiC
immersion in tap Sodium sulfide Na2S
water Sodium fluoride NaF
Magnesium carbonate MgCl ·2MgCO
2
3
Chloride hydroxide hydrate Mg(OH) ·6H O
2
2
Magnesium pyrophosphate Mg P O
7
2 2
Anorthoclase (Na,K)AlSi O
3
8
Alpha cristobalite SiO
2
Sodium hydroxide NaOH
Calcium aluminum oxide Ca Al O SO 4
12
4
6
sulfate
Substance Halite NaCl
found on heat
exchanger
Substance found Alpha quartz SiO 2
beneath paint on
metal surface
Product formed Lepidocrocite g -Fe O ·H O
2
3
2
on automobile Goethite Fe O ·2H O
2
3
2
bumper support
during 3-year
service
Product from Zinc ferrite ZnO·Fe O
2
3
conversion Cobalt ferrite CoO·Fe O
3
2
unit in marine Cobaltous ferrite CoO·Fe O
2
3
environment Halite NaCl
Chromic oxide Cr O
2
3
Nickel, zinc ferrospinel (Ni,Zn)O·Fe O
2
3
Sodium fluothorate Na Th F
3
2 11
Embolite Ag(Cl,Br)
Magnesioferrite MgFe O
2
4
Beryllium palladium BePd
Magnetite Fe O
4
3
Nickel titanium NiTi
Product formed on Botallackite CuCl ·3Cu(OH) ·3H O
2
2
2
copper during 3- Ilvaite Ca(Fe,Mn,Mg) (Fe,Al)
2
month immersion (SiO ) OH
4 2
in tap water
TABLE 2.1 Results of X-Ray Diffraction of Products Found on Specimens Exposed
to Real Environments [1]