Page 41 - Corrosion Engineering Principles and Practice
P. 41
22 C h a p t e r 2 C o r r o s i o n B a s i c s 23
These electrons can participate in chemical reactions and be “stripped”
from the atom, therefore drastically changing its properties. Thus, the
charge of the nucleus is unbalanced and the atom that displays a
positive charge is called an ion.
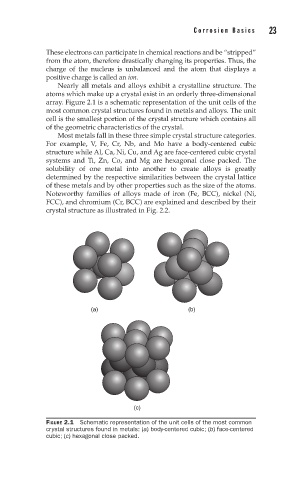
Nearly all metals and alloys exhibit a crystalline structure. The
atoms which make up a crystal exist in an orderly three-dimensional
array. Figure 2.1 is a schematic representation of the unit cells of the
most common crystal structures found in metals and alloys. The unit
cell is the smallest portion of the crystal structure which contains all
of the geometric characteristics of the crystal.
Most metals fall in these three simple crystal structure categories.
For example, V, Fe, Cr, Nb, and Mo have a body-centered cubic
structure while Al, Ca, Ni, Cu, and Ag are face-centered cubic crystal
systems and Ti, Zn, Co, and Mg are hexagonal close packed. The
solubility of one metal into another to create alloys is greatly
determined by the respective similarities between the crystal lattice
of these metals and by other properties such as the size of the atoms.
Noteworthy families of alloys made of iron (Fe, BCC), nickel (Ni,
FCC), and chromium (Cr, BCC) are explained and described by their
crystal structure as illustrated in Fig. 2.2.
(a) (b)
(c)
FIGURE 2.1 Schematic representation of the unit cells of the most common
crystal structures found in metals: (a) body-centered cubic; (b) face-centered
cubic; (c) hexagonal close packed.