Page 39 - Corrosion Engineering Principles and Practice
P. 39
20 C h a p t e r 2 C o r r o s i o n B a s i c s 21
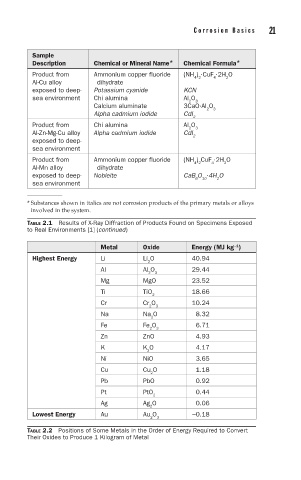
Sample
Description Chemical or Mineral Name* Chemical Formula*
Product from Ammonium copper fluoride (NH ) ·CuF ·2H O
4 2
2
4
Al-Cu alloy dihydrate
exposed to deep- Potassium cyanide KCN
sea environment Chi alumina Al O
2
3
Calcium aluminate 3CaO·Al O
2
3
Alpha cadmium iodide CdI
2
Product from Chi alumina Al O
2
3
Al-Zn-Mg-Cu alloy Alpha cadmium iodide CdI 2
exposed to deep-
sea environment
Product from Ammonium copper fluoride (NH ) CuF ·2H O
4
4 2
2
Al-Mn alloy dihydrate
exposed to deep- Nobleite CaB O ·4H O
6
2
10
sea environment
* Substances shown in italics are not corrosion products of the primary metals or alloys
involved in the system.
TABLE 2.1 Results of X-Ray Diffraction of Products Found on Specimens Exposed
to Real Environments [1] (continued)
Metal Oxide Energy (MJ kg )
-1
Highest Energy Li Li O 40.94
2
Al Al O 29.44
2 3
Mg MgO 23.52
Ti TiO 18.66
2
Cr Cr O 10.24
2 3
Na Na O 8.32
2
Fe Fe O 6.71
2 3
Zn ZnO 4.93
K K O 4.17
2
Ni NiO 3.65
Cu Cu O 1.18
2
Pb PbO 0.92
Pt PtO 0.44
2
Ag Ag O 0.06
2
Lowest Energy Au Au O 3 −0.18
2
TABLE 2.2 Positions of Some Metals in the Order of Energy Required to Convert
Their Oxides to Produce 1 Kilogram of Metal