Page 301 - Design for Environment A Guide to Sustainable Product Development
P. 301
Medical and Pharmaceutical Industries 277
applying “green chemistry” principles during process development.
For example, chemists use electronic lab notebooks that include
green chemistry assessment tools, providing chemists with immedi-
ate feedback on process efficiency and the availability of less hazard-
ous alternative materials.
According to Steve Gillman, Executive Director, Corporate Health,
Safety and Environment, “The most significant health, safety, and envi-
ronmental (HSE) improvements result from our ongoing efforts to
design new products and processes to minimize HSE impacts from the
start. Applying green chemistry and inherently safer design principles
to our process development efforts helps us ‘get it right the first time’.”
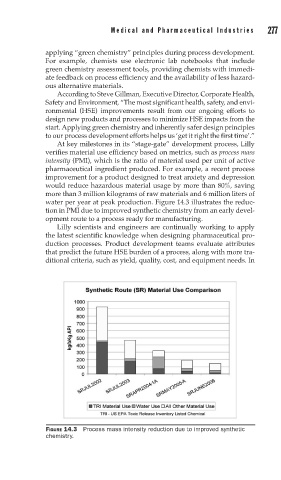
At key milestones in its “stage-gate” development process, Lilly
verifies material use efficiency based on metrics, such as process mass
intensity (PMI), which is the ratio of material used per unit of active
pharmaceutical ingredient produced. For example, a recent process
improvement for a product designed to treat anxiety and depression
would reduce hazardous material usage by more than 80%, saving
more than 3 million kilograms of raw materials and 6 million liters of
water per year at peak production. Figure 14.3 illustrates the reduc-
tion in PMI due to improved synthetic chemistry from an early devel-
opment route to a process ready for manufacturing.
Lilly scientists and engineers are continually working to apply
the latest scientific knowledge when designing pharmaceutical pro-
duction processes. Product development teams evaluate attributes
that predict the future HSE burden of a process, along with more tra-
ditional criteria, such as yield, quality, cost, and equipment needs. In
FIGURE 14.3 Process mass intensity reduction due to improved synthetic
chemistry.