Page 53 - Dust Explosions in the Process Industries
P. 53
26 Dust Explosions in the Process Industries
Dust chemistry influences both thermodynamicsand lunetics, which are also to some
extent coupled. Table 1.1 shows a considerable difference between the amounts of heat
developed per mole of oxygen consumed for various groups of materials. Calcium,
magnesium, and aluminum top the list with 1100-1300 kJ/mole 02.The lowest value is
300 kJ/mole O2for copper and sulfur. It would be expectedthat this differenceis to some
extent reflected in the maximum pressure of explosions, when performed adiabatically
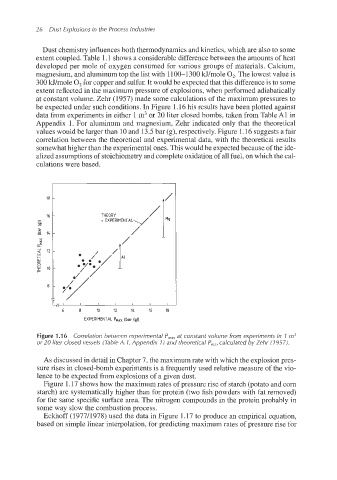
at constant volume. Zehr (1957) made some calculations of the maximum pressures to
be expected under such conditions. In Figure 1.16 his results have been plotted against
data from experiments in either 1 m3or 20 liter closed bombs, taken from Table A1 in
Appendix 1. For aluminum and magnesium, Zehr indicated only that the theoretical
values would be larger than 10 and 13.5bar (g), respectively. Figure 1.16 suggests a fair
correlation between the theoretical and experimental data, with the theoretical results
somewhathigher than the experimentalones. This would be expectedbecause of the ide-
alized assumptionsof stoichiometry and complete oxidationof all fuel, on which the cal-
culations were based.
x c /
2
9 12 -
k-
w
LL
0
y 10 -
I-
8-
-
I I I I I
6 a 10 12 14 16 18
EXPERIMENTAL PMAX [bar [qjl
Figure 1.I 6 Correlation between experimental P,,, at constant volume from experiments in 1 m3
or 20 liter closed vessels (Table A. 1, Appendix 1) and theoretical P,,, calculated by Zehr (1 957).
As discussed in detail in Chapter 7, the maximum rate with which the explosionpres-
sure rises in closed-bomb experiments is a frequently used relative measure of the vio-
lence to be expected from explosions of a given dust.
Figure 1.17 shows how the maximum rates of pressure rise of starch (potato and corn
starch) are systematically higher than for protein (two fish powders with fat removed)
for the same specific surface area. The nitrogen compounds in the protein probably in
some way slow the combustion process.
Eckhoff (1977/1978) used the data in Figure 1.17 to produce an empirical equation,
based on simple linear interpolation, for predicting maximum rates of pressure rise for