Page 71 - Dust Explosions in the Process Industries
P. 71
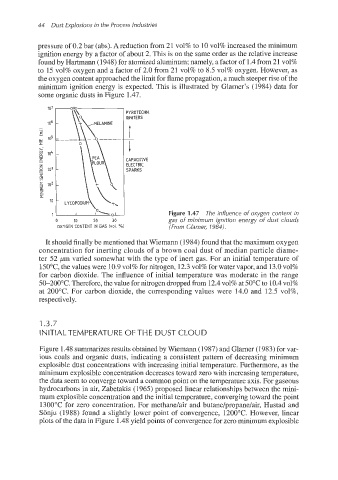
44 Dust Explosions in the Process Industries
pressure of 0.2 bar (abs). Areduction from 21 vol% to 10 vol% increased the minimum
ignition energy by a factor of about 2. This is on the same order as the relative increase
found by Hartmann (1948) for atomized aluminum; namely, a factor of 1.4from 21 vol%
to 15 vol% oxygen and a factor of 2.0 from 21 vol% to 8.5 vol% oxygen. However, as
the oxygen content approachedthe limit for flame propagation, a much steeper rise of the
minimum ignition energy is expected. This is illustrated by Glarner's (1984) data for
some organic dusts in Figure 1.47.
'YROTECHN
,GNlTERS
I
-
1
CAPACITIVE
ELECTRIC
SPARKS
Figure 1.47 The influence of oxygen content in
0 10 20 30 gas of minimum ignition energy of dust ciouds
OXYGEN CONTENT IN GAS IVOl. 701 (From Clarner, 1984).
It should finally be mentioned that Wiemann (1984) found that the maximum oxygen
concentration for inerting clouds of a brown coal dust of median particle diame-
ter 52 pm varied somewhat with the type of inert gas. For an initial temperature of
150"C,the values were 10.9vol% for nitrogen, 12.3vol% for water vapor, and 13.0vol%
for carbon dioxide. The influence of initial temperature was moderate in the range
50-200°C. Therefore, the value for nitrogen dropped from 12.4vol% at 50°C to 10.4vol%
at 200°C. For carbon dioxide, the corresponding values were 14.0 and 12.5 vol%,
respectively.
1.3.7
INITIAL TEMPERATURE OF THE DUST CLOUD
Figure 1.48 summarizesresults obtained by Wiemann (1987) and Glarner (1983)for var-
ious coals and organic dusts, indicating a consistent pattern of decreasing minimum
explosible dust concentrationswith increasing initial temperature. Furthermore, as the
minimum explosible concentration decreases toward zero with increasing temperature,
the data seem to convergetoward a common point on the temperature axis. For gaseous
hydrocarbons in air, Zabetakis (1965) proposed linear relationships between the mini-
mum explosible concentration and the initial temperature,converging toward the point
1300°C for zero concentration. For methane&-and butane/propane/air, Hustad and
Sonju (1988) found a slightly lower point of convergence, 1200°C. However, linear
plots of the data in Figure 1.48yield points of convergencefor zero minimum explosible