Page 265 - Electrical Properties of Materials
P. 265
Piezoelectricity, pyroelectricity, and ferroelectricity 247
Usually two ceramic cylinders are used to double the charge build-up and
hence the voltage across the spark gap. The operating force must be applied
quickly as the charge readily leaks away. The great virtue of PZT is a high
piezoelectric constant, about 200 times greater than quartz, but it is a lossy
–2
dielectric, tan δ is about 10 . You press a spring, which is released to impact
the ceramic so that a gas flow in air is spark-ignited and your fire or cooker
heats up.
10.13.2 Pyroelectricity
As mentioned before, pyroelectrics are a subclass of piezoelectrics. Of the 20
piezoelectric classes of crystals 10 are pyroelectric. The distinguishing fea-
ture of pyroelectrics is that they have a spontaneous dipole moment, P S .The
property that makes them suitable for a variety of applications is the sensit-
ivity of that dipole moment to heat. The pyroelectric coefficient is defined as
p = ∂P s /∂T.
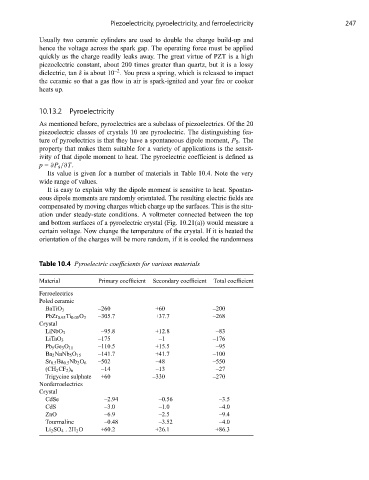
Its value is given for a number of materials in Table 10.4. Note the very
wide range of values.
It is easy to explain why the dipole moment is sensitive to heat. Spontan-
eous dipole moments are randomly orientated. The resulting electric fields are
compensated by moving charges which charge up the surfaces. This is the situ-
ation under steady-state conditions. A voltmeter connected between the top
and bottom surfaces of a pyroelectric crystal (Fig. 10.21(a)) would measure a
certain voltage. Now change the temperature of the crystal. If it is heated the
orientation of the charges will be more random, if it is cooled the randomness
Table 10.4 Pyroelectric coefficients for various materials
Material Primary coefficient Secondary coefficient Total coefficient
Ferroelectrics
Poled ceramic
BaTiO 3 –260 +60 –200
PbZr 0.95 Ti 0.05 O 3 –305.7 +37.7 –268
Crystal
LiNbO 3 –95.8 +12.8 –83
LiTaO 3 –175 –1 –176
Pb 5 Ge 3 O 11 –110.5 +15.5 –95
Ba 2 NaNb 5 O 15 –141.7 +41.7 –100
Sr 0.5 Ba 0.5 Nb 2 O 6 –502 –48 –550
(CH 2 CF 2 ) n –14 –13 –27
Trigycine sulphate +60 –330 –270
Nonferroelectrics
Crystal
CdSe –2.94 –0.56 –3.5
CdS –3.0 –1.0 –4.0
ZnO –6.9 –2.5 –9.4
Tourmaline –0.48 –3.52 –4.0
Li 2 SO 4 .2H 2 O +60.2 +26.1 +86.3