Page 236 - Electromagnetics
P. 236
ε /ε
0
60
40
−ε /ε
0
20
0
7 8 9 10 11 12
log ( f )
10
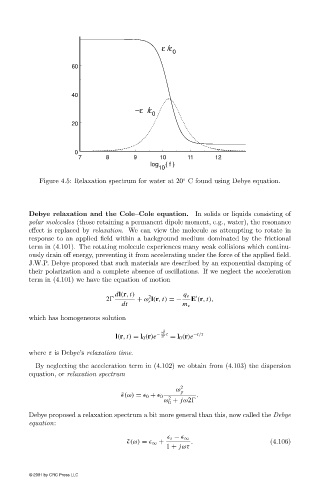
Figure 4.5: Relaxation spectrum for water at 20 C found using Debye equation.
◦
Debye relaxation and the Cole–Cole equation. In solids or liquids consisting of
polar molecules (those retaining a permanent dipole moment, e.g., water), the resonance
effect is replaced by relaxation. We can view the molecule as attempting to rotate in
response to an applied field within a background medium dominated by the frictional
term in (4.101). The rotating molecule experiences many weak collisions which continu-
ously drain off energy, preventing it from accelerating under the force of the applied field.
J.W.P. Debye proposed that such materials are described by an exponential damping of
their polarization and a complete absence of oscillations. If we neglect the acceleration
term in (4.101) we have the equation of motion
dl(r, t) 2 q e
2 + ω l(r, t) =− E (r, t),
r
dt m e
which has homogeneous solution
ω 2 r
t
l(r, t) = l 0 (r)e − 2 = l 0 (r)e −t/τ
where τ is Debye’s relaxation time.
By neglecting the acceleration term in (4.102) we obtain from (4.103) the dispersion
equation, or relaxation spectrum
ω 2 p
˜ (ω) = 0 + 0 2 .
ω + jω2
0
Debye proposed a relaxation spectrum a bit more general than this, now called the Debye
equation:
s − ∞
˜ (ω) = ∞ + . (4.106)
1 + jωτ
© 2001 by CRC Press LLC