Page 152 - Bruno Linder Elementary Physical Chemistry
P. 152
August 18, 2010 11:37 9in x 6in b985-appB Elementary Physical Chemistry
Appendix B
Thermodynamic Data
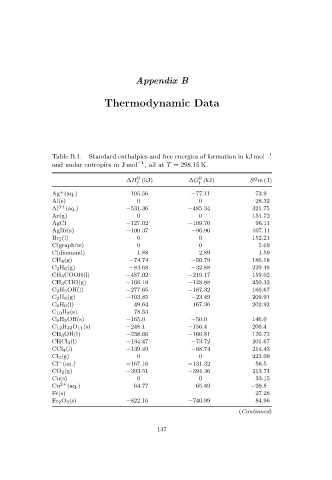
Table B.1. Standard enthalpies and free energies of formation in kJ mol −1
and molar entropies in J mol −1 ,allat T = 298.15 K.
o o o
¯
∆H ¯ f (kJ) ∆G¯ f (kJ) S m(J)
+
Ag (aq.) 105.56 −77.11 73.9
Al(s) 0 0 28.32
Al 3+ (aq.) −531.36 −485.34 321.75
Ar(g) 0 0 154.72
AgCl −127.02 −109.70 96.11
AgBr(s) −100.37 −96.90 107.11
Br 2(l) 0 0 152.21
C(graphite) 0 0 5.69
C(diamond) 1.88 2.89 1.59
CH 4(g) −74.74 −50.79 186.18
C 2H 6 (g) −84.68 −32.88 229.49
CH 3COOH(l) −487.02 −219.17 159.02
CH 3CHO(g) −166.19 −128.88 250.33
C 2H 5OH(l) −277.65 −187.32 160.67
C 3H 8 (g) −103.85 −23.49 209.91
C 6H 6 (l) 49.64 167.36 202.92
C 10 H 8(s) 78.53
C 6H 5 OH(s) −165.0 −50.0 146.0
C 12H 22O 11(s) −248.1 −156.4 200.4
CH 3OH(l) −238.66 −166.81 126.72
CHCl 3(l) −134.47 −73.72 201.67
CCl 4(l) −139.49 −68.74 214.43
Cl 2(g) 0 0 223.09
Cl (aq.) −167.16 −131.32 56.5
−
CO 2(g) −393.51 −394.36 213.74
Cu(s) 0 0 33.15
Cu 2+ (aq.) 64.77 65.49 −99.8
Fe(s) 27.28
Fe 2 O 3(s) −822.16 −740.99 84.96
(Continued)
137