Page 153 - Bruno Linder Elementary Physical Chemistry
P. 153
August 18, 2010 11:37 9in x 6in b985-appB Elementary Physical Chemistry
138 Elementary Physical Chemistry
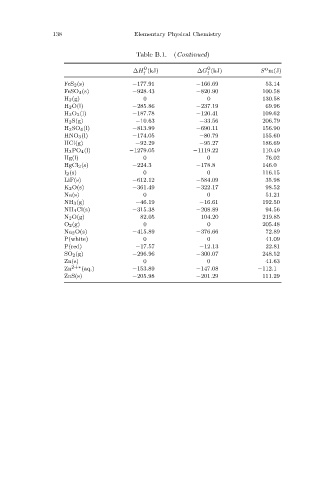
Table B.1. (Continued)
o o o
¯
∆H ¯ f (kJ) ∆G ¯ f (kJ) S m(J)
FeS 2 (s) −177.91 −166.69 53.14
FeSO 4 (s) −928.43 −820.90 100.58
H 2(g) 0 0 130.58
H 2O(l) −285.86 −237.19 69.96
H 2O 2(l) −187.78 −120.41 109.62
H 2S(g) −10.63 −33.56 206.79
H 2SO 4 (l) −813.99 −690.11 156.90
HNO 3(l) −174.05 −80.79 155.60
HCl(g) −92.29 −95.27 186.69
H 3PO 4 (l) −1279.05 −1119.22 110.49
Hg(l) 0 0 76.02
HgCl 2(s) −224.3 −178.8 146.0
I 2(s) 0 0 116.15
LiF(s) −612.12 −584.09 35.98
K 2O(s) −361.49 −322.17 98.52
Na(s) 0 0 51.21
NH 3(g) −46.19 −16.61 192.50
NH 4Cl(s) −315.38 −208.89 94.56
N 2O(g) 82.05 104.20 219.85
O 2(g) 0 0 205.48
Na 2 O(s) −415.89 −376.66 72.89
P(white) 0 0 41.09
P(red) −17.57 −12.13 22.81
SO 2(g) −296.96 −300.07 248.52
Zn(s) 0 0 41.63
Zn 2+∗ (aq.) −153.89 −147.08 −112.1
ZnS(s) −205.98 −201.29 111.29