Page 83 - Bruno Linder Elementary Physical Chemistry
P. 83
August 18, 2010 11:36 9in x 6in b985-ch07 Elementary Physical Chemistry
68 Elementary Physical Chemistry
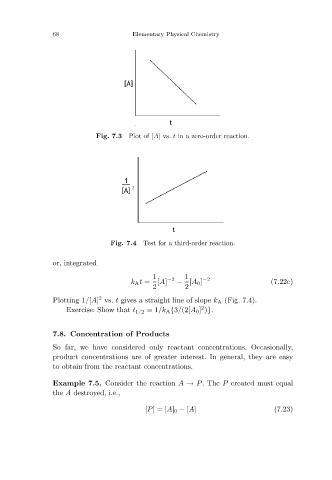
Plot of [A]vs. t in a zero-order reaction.
Fig. 7.3
Test for a third-order reaction.
Fig. 7.4
or, integrated
1 −2 1 −2
k A t = [A] − [A 0 ] (7.22c)
2 2
2
Plotting 1/[A] vs. t gives a straight line of slope k A (Fig. 7.4).
2
Exercise: Show that t 1/2 =1/k A{3/(2[A 0] )}.
7.8. Concentration of Products
So far, we have considered only reactant concentrations. Occasionally,
product concentrations are of greater interest. In general, they are easy
to obtain from the reactant concentrations.
Example 7.5. Consider the reaction A → P.The P created must equal
the A destroyed, i.e.,
[P]= [A] 0 − [A] (7.23)