Page 86 - Bruno Linder Elementary Physical Chemistry
P. 86
August 18, 2010 11:36 9in x 6in b985-ch07 Elementary Physical Chemistry
Chemical Kinetics 71
7.10.1. Collision Theory
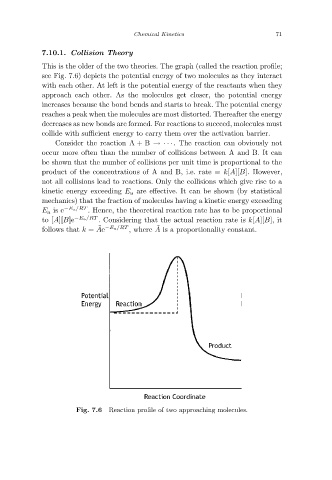
This is the older of the two theories. The graph (called the reaction profile;
see Fig. 7.6) depicts the potential energy of two molecules as they interact
with each other. At left is the potential energy of the reactants when they
approach each other. As the molecules get closer, the potential energy
increases because the bond bends and starts to break. The potential energy
reaches a peak when the molecules are most distorted. Thereafter the energy
decreases as new bonds are formed. For reactions to succeed, molecules must
collide with sufficient energy to carry them over the activation barrier.
Consider the reaction A + B → ··· . The reaction can obviously not
occur more often than the number of collisions between A and B. It can
be shown that the number of collisions per unit time is proportional to the
product of the concentrations of A and B, i.e. rate = k[A][B]. However,
not all collisions lead to reactions. Only the collisions which give rise to a
kinetic energy exceeding E a are effective. It can be shown (by statistical
mechanics) that the fraction of molecules having a kinetic energy exceeding
E a is e −E a /RT . Hence, the theoretical reaction rate has to be proportional
to [A][B]e −E a/RT . Considering that the actual reaction rate is k[A][B], it
˜
˜ −E a /RT
follows that k = Ae ,where A is a proportionality constant.
Reaction profile of two approaching molecules.
Fig. 7.6