Page 87 - Bruno Linder Elementary Physical Chemistry
P. 87
August 18, 2010 11:36 9in x 6in b985-ch07 Elementary Physical Chemistry
72 Elementary Physical Chemistry
˜
˜
The value of A can, in principle, be measured. The measured A is
generally smaller than the one calculated. It is said that the relative
orientation must also be taken into account, and this will give rise to a
˜ −E a /RT
“steric” factor, P A . Hence, k = P AAe .
7.10.2. Activated Complex Theory
This is the modern theory of reaction rate. The collision theory is generally
limited to classical gases. The activated complex theory makes use of
quantum mechanical and statistical mechanical concepts.
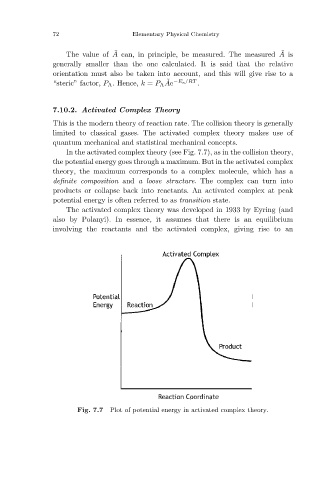
In the activated complex theory (see Fig. 7.7), as in the collision theory,
the potential energy goes through a maximum. But in the activated complex
theory, the maximum corresponds to a complex molecule, which has a
definite composition and a loose structure. The complex can turn into
products or collapse back into reactants. An activated complex at peak
potential energy is often referred to as transition state.
The activated complex theory was developed in 1933 by Eyring (and
also by Polanyi). In essence, it assumes that there is an equilibrium
involving the reactants and the activated complex, giving rise to an
Plot of potential energy in activated complex theory.
Fig. 7.7