Page 84 - Bruno Linder Elementary Physical Chemistry
P. 84
August 18, 2010 11:36 9in x 6in b985-ch07 Elementary Physical Chemistry
Chemical Kinetics 69
(1) In zero-order, from Eq. (7.21b)
[P]= [A 0 ] − [A]= k A t (7.24a)
(2) In first-order, from Eq. (7.18c)
[P]= [A 0 ][1 − exp(−k A t)] (7.24b)
(3) In second-order, from Eq. (7.19c)
1/[A] − 1/[A 0]= k At
2
[P]= k A t[A 0 ] /{1+ k A t[A 0 ]} (7.24c)
7.9. Temperature-Dependent Reaction Rates.
The Arrhenius Equation
Arrhenius found that many reactions obey the Law
˜
ln k =ln A − E a/RT
˜ −E a/RT
k = Ae (7.25)
˜
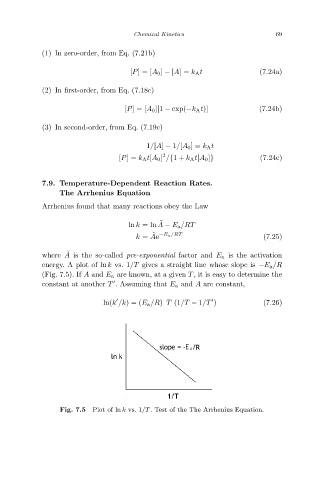
where A is the so-called pre-exponential factor and E a is the activation
energy. A plot of ln k vs. 1/T gives a straight line whose slope is −E a/R
(Fig. 7.5). If A and E a are known, at a given T , it is easy to determine the
constant at another T . Assuming that E a and A are constant,
ln(k /k)= (E a /R) T (1/T − 1/T ) (7.26)
Plot of ln k vs. 1/T. Test of the The Arrhenius Equation.
Fig. 7.5