Page 131 - Elements of Chemical Reaction Engineering 3rd Edition
P. 131
Sec. 3.3 Stoichiometric Table 103
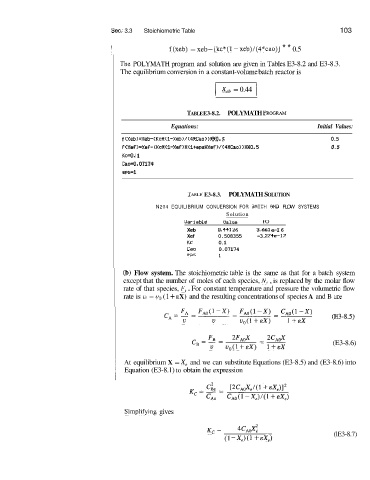
I f(xeb) = xeb- [kc*(l -xeb)/(4*cao)] * * 0.5
The, POLYMATH program and solution are given in Tables E3-8.2 and E3-8.3.
The equilibrium conversion in a constant-volume batch reactor is
I X,, = 0.44 1
TABLE E3-8.2. POLYMATH F’ROGRAM
Equations: Initial Values:
f IXeb)=Xeb-(KcX(l-Xeb)/I4XCao) HXO. 5 0.5
fIXef)=Xef-IKcW(1-Xef)X(l+epsWXef~~(4XCao))XX0.5 0.5
Kc=O. 1
Can=0.07174
eps=l
TADLE E3-8.3. POLYMATH SOLUTION
N204 EQUILIBRIUM CONUERSION FOR BATCI-I AN0 FLOW SYSTEMS
Solution
Uariable Ualue io
Xeb 0.441 26 3.661 e-I 6
Xef 0.508355 -3. %74e-17
Kc 0.1
Can 0.07174
eps 1
(b) Flow system. The stoichiometric table is the same as that for a batch system
except that the number of moles of each species, N, , is replaced by the molar flow
rate of that species, F, . For constant temperature and pressure the volumetric flow
rate is u = uo (1 + EX) and the resulting concentrations of species A and B ire
(E3-8.6)
1 At equilibrium X = X, and we can substitute Equations (E3-8.5) and (E3-8.6) into
I Equation (E3-8.1) to obtain the expression
Si-mplifying gives
KC = 4cA&2 (lE3-8.7)
(1 -X,)(l +ex>