Page 134 - Elements of Chemical Reaction Engineering 3rd Edition
P. 134
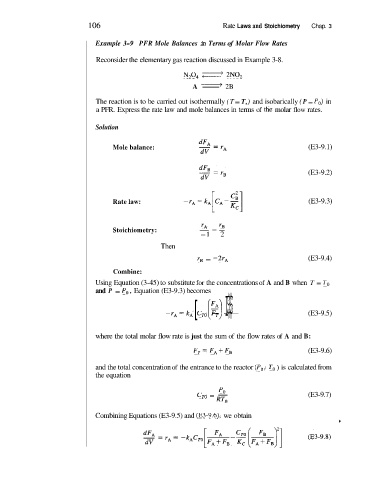
106 Rate Laws and Stoichiometry Chap. 3
Example 3-9 PFR Mole Balances in Terms of Molar Flow Rates
Reconsider the elementary gas reaction discussed in Example 3-8.
N,O, 2N0,
A -
2B
+
The reaction is to be carried out isothermally (T = T,) and isobarically (P = Po) in
a PFR. Express the rate law and mole balances in terms of the molar flow rates.
Solution
Mole balance: (E3-9.1)
(E3-9.2)
Rate law: (E3-9.3)
‘A - ‘B
Stoichiometry: - --
-1 2
Then
r, = -2r, (E3-9.4)
Combine:
Using Equation (3-45) to substitute for the concentrations of A and B when T = To
and P = Po, Equation (E3-9.3) becomes
$$$I
-rA=kd [ C,, (:) - -- (E3-9.5)
where the total molar flow rate is just the sum of the flow rates of A and B:
F,= FAi-FB (E3-9.6)
and the total concentration of the entrance to the reactor (Po, To ) is calculated from
the equation
PO
c,, = - (E3-9.7)
RTO
Combining Equations (E3-9.5) and (E3-9.6), we obtain