Page 186 - Elements of Chemical Reaction Engineering 3rd Edition
P. 186
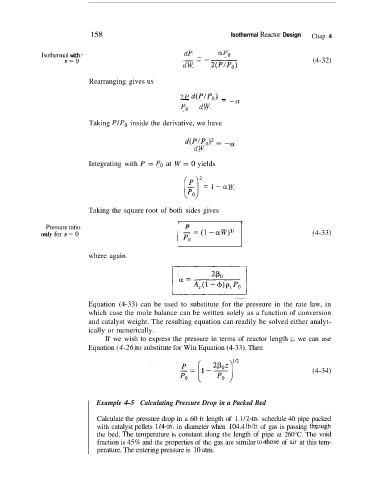
158 Isothermal Reactor Design Chap. 4
Isothermal with __ -- aP0
dP -
E=O (4-32)
dW 2(P/Po)
Rearranging gives us
2P d(PIP0)
--- - -a
Po dW
Taking PIPo inside the derivative, we have
-
d(P/Po)’ - -a
dW
(1 2 =1-aW
Integrating with P = Po at W = 0 yields
Taking the square root of both sides gives
I I
Pressure ratio P
only for E = 0 (4-33)
where again
Equation (4-33) can be used to substitute for the pressure in the rate law, in
which case the mole balance can be written solely as a function of conversion
and catalyst weight. The resulting equation can readily be solved either analyt-
ically or numerically.
If we wish to express the pressure in terms of reactor length z, we can use
Equation (4-26) to substitute for Win Equation (4-33). Then
(4-34)
Example 4-5 Calculating Pressure Drop in a Packed Bed
Calculate the pressure drop in a 60 ft length of 1 1/2-in. schedule 40 pipe packed
with catalyst pellets 114-h. in diameter when 104.4 lb/h of gas is passing thzough
the bed, The temperature is constant along the length of pipe at 260°C. The void
fraction is 45% and the properties of the gas are similar to&ose of air at this tem-
perature. The entering pressure is 10 atm.