Page 448 - Elements of Chemical Reaction Engineering Ebook
P. 448
Chap. 7' Questions and Problems 41 9
Additional information:
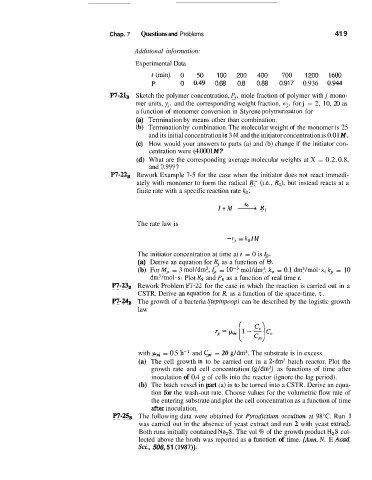
Experimental Data
t(min) 0 50 100 200 400 700 1200 1600
P 0 0.49 0.68 0.8 0.88 0.917 0.936 0.'944
Sketch the polymer concentration, P7, mole fraction of polymer with j mono-
mer units, y,, and the corresponding weight fraction, w,, for j = 2, 10, 20 as
a function of monomer conversion in Styrene polymerizatio-n for
(a) Termination by means other than combination.
(b) Termination by combination. The molecular weight of the monomer is 25
and its initial concentration is 3 M and the initiator concentration is 0.01 M.
(c) How would your answers to parts (a) and (b) change if the initiator con-
centration were (4.0001 M?
(d) What are the corresponding average molecular weights at X = 0.2, 0.8,
and 0.999?
Rework Example 7-5 for the case when the initiator does not react immedi-
ately with monomer to form the radical R; (i.e., R1), but instead reacts at a
finite rate with a specific reaction rate ko:
I+M ko R,
The rate law is
- r, = k, IM
The initiator concentration at time at t = 0 is Io.
(a) Derive an equation for Rl as a function of 0.
(b) For M, = 3 mol/dm3, I, = mol/dm3, k, = 0.1 dm3/mol.s, kp = 10
dm3/mol.s. Plot R8 and Ps as a function of real time t.
W-23, Rework Problem P7-22 for the case in which the reaction is carried out in a
CSTR. Derive an 6quation for R, as a function of the space-time, 7.
W-2% The growth of a bacteria Stepinpoopi can be described by the logistic growth
law
with p,, = 0.5 h-l and C, = 20 g/dm3. The substrate is in excess.
(a) The cell growth is to be carried out in a 2-dm3 batch reactor. Plot the
growth rate and cell concentration (g/dm3) as functions of time after
inoculation of 0.4 g of cells into the reactor (ignore the lag period).
(b) The batch vessel in part (a) is to be turned into a CSTR. Derive an equa-
tion for the wash-out rate. Choose values for the volumetric flow rate of
the entering substrate and plot the cell concentration as a function of time
after inoculation.
W-2SB The following data were obtained for Pyrodictium occulturn at 98°C. Run 1
was carried out in the absence of yeast extract and run 2 with yeast extra&
Both runs initially contained Na,S. The vol % of the growth product H,S col-
lected above the broth was reported as 8 functioh of time. [Ann. N. E ,A.cad.
Sci., 506, 51 (1987)l.