Page 447 - Elements of Chemical Reaction Engineering Ebook
P. 447
41 8 Nonelementary Reaction Kinetics Chap. 7
The entering concentration of monomer and initiator areMo and Io, respectively.
(a) Derive an equation involving the monomer concentration and only the
variables kp, kl, Io, t (i.e., z = V/u), and MQ
(b) Derive an equation of the radical concentration as a function of space time, z .
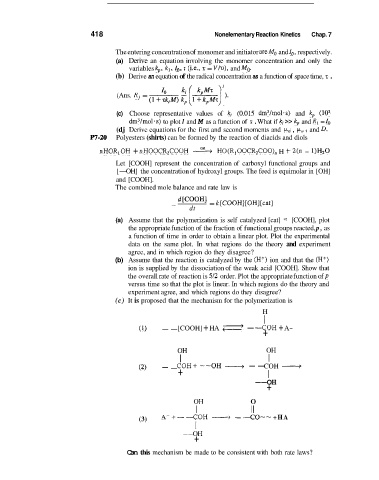
(Ans. Rj =
(c) Choose representative values of k, (0.015 dm3/mol.s) and kp (lo3
dm3/mol-s) to plot I and M as a function of z . What if k, >> kp and R1 = Io
(dj Derive equations for the first and second moments and pn , pw, and D.
W-20 Polyesters (shirts) can be formed by the reaction of diacids and diols
nHORIOH + nHOOCRzCOOH 4 HO(R,00CR2COO),, H + 2(n - 1)H20
Let [COOH] represent the concentration of carboxyl functional groups and
[-OH] the concentration of hydroxyl groups. The feed is equimolar in [OH]
and [COOH].
The combined mole balance and rate law is
- d[CooH1 = k{COOH][OH][cat]
dt
(a) Assume that the polymerization is self catalyzed [cat] = [COOH], plot
the appropriate function of the fraction of functional groups reacted, p, as
a function of time in order to obtain a linear plot. Plot the experimental
data on the same plot. In what regions do the theory and experiment
agree, and in which region do they disagree?
(b) Assume that the reaction is catalyzed by the (H+) ion and that the (H+)
ion is supplied by the dissociation of the weak acid [COOH]. Show that
the overall rate of reaction is 5/2 order. Plot the appropriate function of p
versus time so that the plot is linear. In which regions do the theory and
experiment agree, and which regions do they disagree?
(c) It is proposed that the mechanism for the polymerization is
H
I
[COOH] + HA <I
-COH
(1) -- - + + A -
OH
OH -- COH -
I I
COH+ --OH
(2) - - I
+
--OH
+
OH 0
I I1
(3) A- +- -COH __+ - -CO-- +HA
I
--OH
+
Can this mechanism be made to be consistent with both rate laws?