Page 511 - Elements of Chemical Reaction Engineering Ebook
P. 511
Sec. 8.5 Nonadiabatic Reactor Operation: Oxidation of Sulfur Dioxide Example 481
' - 1
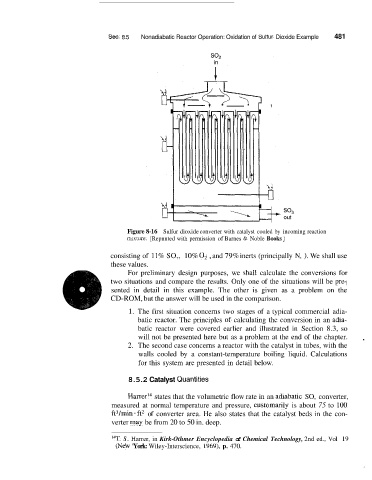
Figure 8-16 Sulfur dioxide converter with catalyst cooled by incoming reaction
nuxture. [Repnnted with permission of Barnes & Noble Books ]
consisting of 11% SO,, 10% 0, , and 79% inerts (principally N, >. We shall use
these values.
For preliminary design purposes, we shalI calculate the conversions for
two situations and compare the results. Only one of the situations will be lpre-
sented in detail in this example. The other is given as a problem on the
CD-ROM, but the answer will be used in the comparison.
1. The first situation concerns two stages of a typical commercial adia-
batic reactor. The principles of calculating the conversion in an aldia-
batic reactor were covered earlier and illustrated in Section 8.3, so
will not be presented here but as a problem at the end of the chapter.
2. The second case concerns a reactor with the catalyst in tubes, with the '
walls cooled by a constant-temperature boiling liquid. Calculations
for this system are presented in detail below.
8.5.2 Catalyst Quantities
Harrer14 states that the volumetric flow rate in an a&abatic SO, converter,
measured at normal temperature and pressure, customarily is about 75 to 100
ft3/min-ft2 of converter area. He also states that the catalyst beds in the con-
verter rnay be from 20 to 50 in. deep.
I4T. S. Harrer, in Kirk-Othmer Encyclopedia of Chemical Technology, 2nd ed., Vol 19
(Neh 'York: Wiley-Interscience, 1969), p. 470.