Page 523 - Elements of Chemical Reaction Engineering Ebook
P. 523
Sec. 8.6 Multiple Steady States 493
Finally, substituting for k in terms of the Arrhenius equation, we obtain
(13-72)
Note that equations analogous to Equation (8-71) for G(T) can be derived for
other reaction orders and for reversible reactions simply by solving the CSTR
mole balance for X. For example, for the second-order liquid-phase reaction
2nd order reaction
and the corresponding heat generated is
-AH:, [(2rCAoAe-EIRT+ 1) - ,,/&CA,Ae-E/RT + 11
G(T) = (8-73)
~zC~~A~-~/~~
Pit very low temperatures, the second term in the denominator of Equation
(8-72) can be neglected, so that G(T) varies as
Low T @(T) = -AHi,zAe-E/R1*
(Recall AH& means the heat of reaction is evaluated at TR.)
At very high temperatures, the second term in the denominator dominates and
G(T) is reduced to
High T C(T) = -AH:,
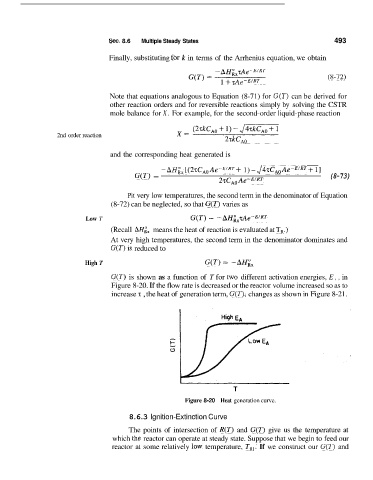
G(T) is shown as a function of T for two different activation energies, E,, in
Figure 8-20. If the flow rate is decreased or the reactcir volume increased so as to
increase T , the heat of generation term, G(T), changes as shown in Figure 8-21.
T
Figure 8-20 Heat generation curve.
8.6.3 Ignition-Extinction Curve
The points of intersection of R(T) and G(T) give us the temperature at
which the reactor can operate at steady state. Suppose that we begin to feed our
reactor at some relatively lo* temperature, To,. If we construct our G(T) and