Page 526 - Elements of Chemical Reaction Engineering Ebook
P. 526
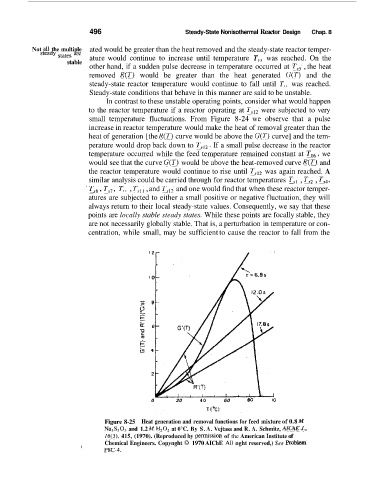
496 Steady-State Nonisothermal Reactor Design Chap. 8
Not all the multiple ated would be greater than the heat removed and the steady-state reactor temper-
steady are ature would continue to increase until temperature T,, was reached. On the
stable
other hand, if a sudden pulse decrease in temperature occurred at T,, , the heat
removed R(T) would be greater than the heat generated G(T) and the
steady-state reactor temperature would continue to fall until T,, was reached.
Steady-state conditions that behave in this manner are said to be unstable.
In contrast to these unstable operating points, consider what would happen
to the reactor temperature if a reactor operating at Tr12 were subjected to very
small temperature fluctuations. From Figure 8-24 we observe that a pulse
increase in reactor temperature would make the heat of removal greater than the
heat of generation [the R(T) curve would be above the G(T) curve] and the tem-
perature would drop back down to TS,*. If a small pulse decrease in the reactor
temperature occurred while the feed temperature remained constant at To,, we
would see that the curve G(T) would be above the heat-removed curve R(T) and
the reactor temperature would continue to rise until Ts,2 was again reached. A
similar analysis could be carried through for reactor temperatures TS, , T,, , TF4,
' Ts6, Tr7, T,, , T,,, , and T,,, and one would find that when these reactor ternper-
atures are subjected to either a small positive or negative fluctuation, they will
always return to their local steady-state values. Consequently, we say that these
points are locally stable steady states. While these points are focally stable, they
are not necessarily globally stable. That is, a perturbation in temperature or con-
centration, while small, may be sufficient to cause the reactor to fall from the
IO
0
6
4
2
0 20 40 60 eo IO
Figure 8-25 Heat generation and removal functions for feed mixture of 0.8 M
Na2S203 and 1.2 M H,02 at 0°C. By S. A. Vejtasa and R. A. Schmitz, AlChEJ.,
16(3), 415, (1970). (Reproduced by permission of the American Institute of
Chemical Engineers. Copynght 0 1970 AIChE A11 nght reserved,) See Pmblem
I
P8C-4.