Page 57 - Elements of Chemical Reaction Engineering Ebook
P. 57
28 Mole Balances Chap. 1
e Represents mantains
or hills
LA. YO Wind from
Side view
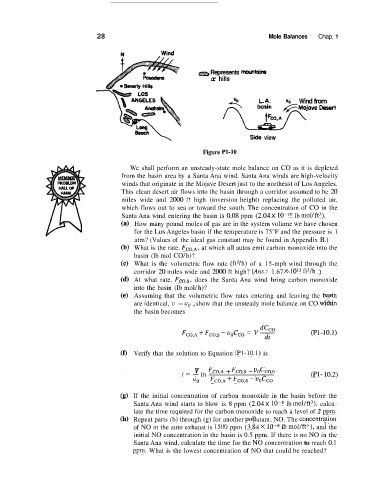
Figure P1-10
We shall perform an unsteady-state mole balance on CO as it is depleted
from the basin area by a Santa Ana wind. Santa Ana winds are high-velocity
winds that originate in the Mojave Desert just to the northeast of Los Angeles.
This clean desert air flows into the basin through a corridor assumed to be 20
miles wide and 2000 ft high (inversion height) replacing the polluted air,
which flows out to sea or toward the south. The concentration of CO in the
Santa Ana wind entering the basin is 0.08 ppm (2.04 X lb mol/ft3).
How many pound moles of gas are in the system volume we have chosen
for the Los Angeles basin if the temperature is 75°F and the pressure is 1
atm? (Values of the ideal gas constant may be found in Appendix B.)
What is the rate, FCO,A, at which all autos emit carbon monoxide into the
basin (lb mol CO/ h)?
What is the volumetric flow rate (ft3/h) of a 15-mph wind through the
corridor 20 miles wide and 2000 ft high? (Am.: 1.67 ft3/h .)
At what rate, Fco,s, does the Santa Ana wind bring carbon monoxide
into the basin (Ib mol/ h)?
are identical, u = uo , show that the unsteady mole balance on CO /
Assuming that the volumetric flow rates entering and leaving the ba 'n
ithm
the basin becomes
(Pl-10.1)
Verify that the solution to Equation (Pl-10.1) is
v
t=-ln FC0.A + FC0.S - yoCc0,o (PI- 10.2)
uO FCO,A + FCO,S - LOcCO
If the initial concentration of carbon monoxide in the basin before the
Santa Ana wind starts to blow is 8 ppm (2.04 X lo-* lb mol/ft3), calcu-
late the time required for the carbon monoxide to reach a level of 2 ppm.
Repeat parts (b) through (g) for another PO lutant, NO. The concentr?tion
of NO in the auto exhaust is 150U ppm (3. B 4 X Ib mol/ft3), and the
initial NO concentration in the basin is 0.5 ppm. If there is no NO in the
Santa Ana wind, calculate the time for the NO concentration to reach 0.1
ppm. What is the lowest concentration of NO that could be reached?