Page 71 - Elements of Chemical Reaction Engineering Ebook
P. 71
42 Conversion and Reactor Sizing Chap. 2
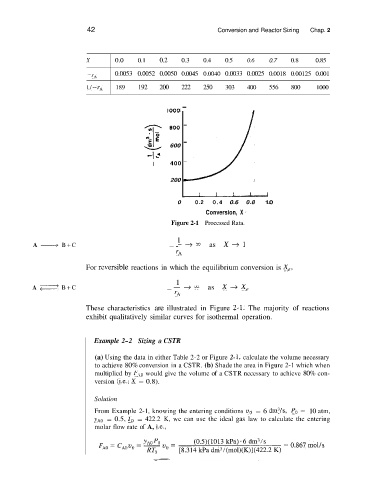
X 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.85
-rA 0.0053 0.0052 0.0050 0.0045 0.0040 0.0033 0.0025 0.0018 0.00125 0.001
l/-rA 189 192 200 222 250 303 400 556 800 1000
1000 -
-
eo0
-
600
400 -
200
0 0.2 0.4 0.6 0.8 1.0
Conversion, X
Figure 2-1 Processed Rata.
A + B+C -_ '--+m as X-+I
rA
For rmersible reactions in which the equilibrium conversion is X,,
A e - --+m as X+X,
1
B+C
rA
These characteristics are illustrated in Figure 2-1. The majority of reactions
exhibit qualitatively similar curves for isothermal operation.
Example 2-2 Sizing a CSTR
(a) Using the data in either Table 2-2 or Figure 2- 1, calculate the volume necessary
to achieve 80% conversion in a CSTR. (b) Shade the area in Figure 2-1 which when
multiplied by F,,,,, would give the volume of a CSTR necessary to achieve 80% con-
version (Le., X = 0.8).
Solution
From Example 2-1, knowing the entering conditions u,, = 6 dm3/s, Po = 10 atm,
yAO = 0.5, To = 422.2 K, we can use the ideal gas law to calculate the entering
molar flow rate of A, Le.,